Abstract
Background
The basis of the less severe clinical presentation of coronavirus disease 2019 (COVID-19) in children as compared with adults remains incompletely understood. Studies have suggested that a more potent boosting of immunity to endemic common cold coronaviruses (HCoVs) may protect children.
Methods
To test this hypothesis, we conducted a detailed analysis of antibodies induced by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in children aged 2 months to 14 years.
Results
Younger children had higher titers of antibodies to SARS-CoV-2 receptor binding domain (RBD), S1 but not S2 domain, and total spike (S) protein, higher avidity RBD immunoglobulin G, and higher titers of neutralizing and complement-activating antibodies as compared with older children. In contrast, older children had higher titers of antibodies to HCoVs, which correlated with antibodies to the SARS-CoV-2 S2 domain but not with neutralizing or complement-activating antibodies.
Conclusions
These results reveal a unique capacity of young children to develop effector antibody responses to SARS-CoV-2 infection independently of their immunity to HCoVs.
Keywords: COVID-19, antibodies, children, endemic coronaviruses, SARS-CoV-2
Since the beginning of the pandemic, children have been underrepresented among cases of coronavirus disease 2019 (COVID-19) [1–3]. Most infected children are asymptomatic or present a milder disease than adults. A small proportion develop multisystem inflammatory syndrome (MIS-C) [4, 5]. Between December 2019 and September 2021, children under 5 represented 1.8% of global cases, and older children and young adolescents (5 to 14 years) represented 6.3% of global cases [6]. The mild clinical presentation of COVID-19 in children contrasts with their high susceptibility to severe infections caused by other respiratory pathogens, such as respiratory syncytial virus or influenza [7–10]. The current understanding indicates that immunity to infectious pathogens in childhood involves a fine balance between immune effector functions and immune regulation preventing immunopathology [8, 11]. Evidence suggests that this balance may be particularly efficient in children infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [5].
Innate immunity involving type I interferons is essential for the control of SARS-CoV-2 replication during the early phase of the infection, whereas excessive innate immune responses play a pathogenic role later in the course of COVID-19 [12]. Children with mild COVID-19 develop robust innate immune responses that resolve faster than in adults, suggesting efficient control of inflammatory responses [5, 13, 14]. On the other hand, adaptive immune responses are essential to clear SARS-CoV-2 infection and provide immunity to re-infections with homologous SARS-CoV-2 or variants thereof [15]. Children are able to develop robust and sustained T-cell and B-cell responses to SARS-CoV-2 infection [5, 16]. Several studies indicate that the magnitude of antibody and T-cell responses to mild COVID-19 is higher in children as compared with adults [17, 18]. It was proposed that these higher adaptive immune responses to SARS-CoV-2 may be related to higher preexisting immunity to endemic common cold coronaviruses (HCoVs) in children as compared with adults [5, 19, 20]. Indeed, SARS-CoV-2 cross-reactive T cells and antibodies have been detected in samples collected before the COVID-19 pandemic, and upregulation, or back-boosting, of these responses was observed following SARS-CoV-2 infection [21]. Evidence for a role of back-boosting of immunity to HCoVs was recently provided by Dowell et al., who showed higher levels of antibodies to beta HCoVs (OC43 and HKU-1) in children as compared with adults following mild or asymptomatic SARS-CoV-2 infection [19]. These higher levels of antibodies to beta HCoVs were associated with higher antibody and T-cell responses to the SARS-CoV-2 spike protein. In contrast, antibody levels to alpha HCoVs (NL63 and 229E), which have a lower degree of homology with SARS-CoV-2 than beta HCoVs, were similar in children and adults, further supporting a role for back-boosting. The possibility that children may be protected from COVID-19 by preexisting immunity to beta HCoVs has important implications for our understanding of immunity to SARS-CoV-2 and for immunization strategies [20].
Yet, important knowledge gaps remain to be filled to define these implications more clearly. First, it may be expected that immunity to HCoVs increases during childhood and that immunity to HCoVs may therefore not explain the reduced disease severity observed in younger children as compared with older children [6]. Second, as it is the S2 domain of beta HCoVs S protein that has the strongest homology with SARS-CoV-2 S protein, it could be expected that SARS-CoV-2 infection would back-boost S2-specific antibodies that are predominantly non-neutralizing rather than antibodies targeting the receptor binding domain (RBD) with a greater neutralization likelihood [21]. In order to explore these knowledge gaps, we performed a comprehensive analysis of the antibody response to SARS-CoV-2 and to HCoVs in a cohort of children aged 2 months to 14 years analyzed at a standardized time point following infection.
METHODS
Study Population
The study was conducted at the Saint-Pierre University Hospital, Brussels. The hospital clinical laboratory provided the list of all children aged <15 years who were tested for SARS-CoV-2 by diagnostic reverse transcription polymerase chain reaction (RT-PCR) using a nasopharyngeal swab between March 1 and December 31, 2020. Indications for SARS-CoV-2 testing evolved during this period, following the recommendations of the Belgian Public Health Institute. March 1 to May 3: Symptoms possibly related to SARS-CoV-2 infection and recent travel in a high-risk country or high-risk contact with a COVID-19 case. May 4 to July 8: At least 1 major symptom (cough, dyspnea, chest pain, anosmia, or dysgeusia) or 2 minor symptoms (fever, myalgia, asthenia, rhinitis, sore throat, anorexia, diarrhea, headache) possibly related to SARS-CoV-2 infection or high risk contact with a COVID-19 case. July 9 to August 14: Symptomatic children under 3 years of age were tested only if other cases were reported in the same nursery school. August 15 to December 31: Symptomatic children under 6 years of age were tested only if other cases were reported in the same school. In addition, all children requiring hospitalization for any cause were tested for SARS-CoV-2 by RT-PCR. Over the 10-month period, 2881 nasopharyngeal swabs were performed, and 136 (4.8%) were RT-PCR positive (Supplementary Table 1). The parents or legal guardians of children with history of a positive SARS-CoV-2 RT-PCR test were contacted by phone and were invited to participate in the study. Parents of 49 of the 136 children consented to their children’s participation in the study and signed a written informed consent. A blood sample was then collected from the child between 3 and 5 months after the diagnosis of SARS-CoV-2 infection. Clinical records were retrieved, and children were included in 1 of the 3 study groups depending on their clinical symptoms at the time of the positive SARS-CoV-2 RT-PCR (Table 1). Group 1 included asymptomic children, group 2 included children with symptoms compatible with COVID-19 who did not require hospitalization, and group 3 included children who had symptoms requiring hospitalization.
Table 1.
Characteristics of the 48 Children Analyzed for Antibody Responses
| Group 1 (Asymptomatic) (n = 10) | Group 2 (Mild) (n = 32) | Group 3 (Hospitalized) (n = 6) | |
|---|---|---|---|
| Age at time of infection, mo | |||
| Median (95% CI) | 112 (34–130) | 133 (84–142) | 76 (2–160) |
| Age, No. (%) | |||
| <1 y | 2 (20) | 8 (25) | 2 (33) |
| 1–10 y | 4 (40) | 5 (16) | 2 (33) |
| >10 y | 4 (40) | 19 (59) | 2 (33) |
| Female, No. (%) | 5 (50) | 14 (44) | 2 (33) |
| Interval between swab and blood test, median (95% CI), d | 107 (92–133) | 120 (110–127) | 126 (93–151) |
| Time of presentation of COVID, No. (%) | |||
| Mar–Apr 2020 | 2 (20) | 1 (3) | 1 (17) |
| May–Jun 2020 | 1 (10) | 3 (9) | 1 (17) |
| Jul–Aug 2020 | 2 (20) | 3 (9) | 0 |
| Sept–Oct 2020 | 5 (50) | 20 (63) | 2 (33) |
| Nov–Dec 2020 | 0 | 5 (16) | 2 (33) |
| Clinical presentation | |||
| Fever | 1a | 26 | 5 |
| Respiratory symptoms | - | 22 | 4 |
| GI symptoms | - | 12 | 2 |
| Headache | - | 11 | 4 |
| Seizure | - | 0 | 0 |
| Skin rash | - | 2 | 0 |
| Anosmia/agueusia | - | 1 | 1 |
| MIS-C | - | 0 | 3b |
| Biological parametersc | n = 3 | n = 6 | n = 6 |
| Median (range) | … | … | … |
| WBC, /μL | 12 750 (5100–16 110) | 10 385 (3950–23 680) | 7155 (4760–15 290) |
| Hemoglobin, g/dL | 10.8 (9.6–13.3) | 12.3 (10.7–14.4) | 11.2 (8.7–12.5) |
| CRP, mg/L | 99.4 (0.5–198.2a) | 3.6 (0.5–54.3) | 121.8 (0.5–201.0) |
Abbreviations: COVID, coronavirus; CRP, C-reactive protein; GI, gastrointestinal; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; WBC, white blood cell; MIS-C: multisystem inflammatory syndrome in children.
Child with increased CRP hospitalized for acute pyelonephritis.
Two admitted to intensive care unit.
Biological parameters were tested for a restricted number of children with asymptomatic or mild SARS-CoV-2 infection.
Patient Consent
The parents or legal guardians of children with history of a positive SARS-CoV-2 RT-PCR test were contacted by phone and were invited to participate in the study. Parents of 49 of the 136 children consented to their children’s participation in the study and signed a written informed consent. The study and its design have been approved by the ethics committee of Saint-Pierre University Hospital, Brussels.
Antigens
The following SARS-CoV-2 antigens were used: homotrimerized spike protein, S (kindly provided by Arnaud Didierlaurent, University of Geneva, Switzerland), receptor-binding domain of the spike protein (RBD; SinoBiological #40592-VNAH), subunit S1 (SinoBiological #40591-V08H), and subunit S2 (SinoBiological #40590-V08B). The following HCoV spike proteins were used: HKU1 (SinoBiological #40606-V08B), NL63 (SinoBiological #40604-V08B), OC43 (SinoBiological #40607-V08B), and 229E (SinoBiological #40605-V08B).
Titers of SARS-CoV-2 and HCoVs-Specific Antibodies
Titers of antigen-specific antibody isotypes and subclasses were assessed using a 96-well-based customized multiplexed Luminex assay. Briefly, antigens were coupled by covalent NHS-ester linkages via EDC and NHS (Pierce #77149 and #24520, respectively) to fluorescent carboxyl-modified microspheres (Luminex). Antigen-coupled microspheres were incubated for 2 hours at room temperature (RT) at 700-rpm orbital shaking, with serum samples at the appropriate dilution: 1:500 for immunoglobulin (Ig)G, IgG1, and IgG3; 1:200 for IgG2, IgA, IgA1, and IgA2; and 1:10 for IgG4 titration. Antigen-specific antibody titers were detected using 0.65 µg/mL of PE-coupled detection antibodies for each isotype and subclass, including IgG (Biolegend # 409304), IgG1 (Southern Biotech #9052-09), IgG2 (Southern Biotech #9070-09), IgG3 (Southern Biotech #9210-09), IgG4 (Southern Biotech #9200-09), IgA (Southern Biotech #2050-09), IgA1 (Southern Biotech #9130-09), and IgA2 (Southern Biotech #9140-09). Antigen-antibody reactions were read on a BioPlex-200 (Bio-Rad), and the results were expressed as median fluorescence intensity (MFI).
Complement Deposition Assay
Antibody-dependent complement deposition (ADCD) was quantified using a 96-well-based customized multiplexed Luminex assay, as described previously [13]. Bulk IgG was purified and separated from other serum proteins using Melon Gel resin according to the manufacturer's instructions (Thermo Scientific #45208). Purified IgG at a 1:75 dilution was incubated with antigen-coupled beads for 2 hours at 37°C on a 700-rpm orbital shaker. After incubation, each sample was incubated with human complement serum (Sigma #S1764) at a concentration of 1:50 at 37°C for 30 minutes. Samples were then incubated at RT for 30 minutes with biotinylated monoclonal antihuman C3d (Quidel #207) at a 1-µg/mL final concentration. Finally, 1 µg/mL of Streptavidin-RPE (Prozyme #PJ31S) was added to each well and incubated at 37°C in the dark for 1 hour. Complement deposition was determined on a BioPlex-200 (Bio-Rad) and measured as median fluorescence intensity (MFI). Assays performed without IgG and with heat-inactivated human complement serum were used as negative controls.
Neutralization Assay
SARS-CoV-2-neutralizing antibodies (nAbs) were quantified as previously reported [22]. Briefly, serial dilutions of heat-inactivated serum (1/50–1/25600 in EMEM supplemented with 2 mM of L-glutamine, 100 U/mL–100 μg/mL of penicillin–streptomycin, and 2% fetal bovine serum) were incubated for 1 hour (37°C, 7% CO2) with 3xTCID100 of wild-type (WT) Wuhan strain (2019-nCoV-Italy-INMI1, reference 008V-03893) or VLD20211207 (B.1.1.529, Omicron BA1). One hundred microliters of sample-virus mixtures and virus/cell controls was added to 100 µL of Vero cells (18 000 cells/well) in a 96-well plate and incubated for 5 days (37°C, 7% CO2). The CPE caused by viral growth was scored microscopically. The Reed-Muench method was used to calculate the nAb titer that reduced the number of infected cells by 50% (NT50), which was used as a proxy for the nAb concentration in the sample. In accordance with WHO guidance, an internal reference standard composed of a pool of sera from naturally infected and vaccinated adults is included in each nAb assay run. This internal standard was calibrated against the International Standard 21/234 (NIBSC), and NT50 values were recalculated to IU/mL for each variant. Against the B.1 variant, the internal standard had an NT50 of 333 IU/mL.
SARS-CoV-2 RBD-Specific Antibody Avidity
Bio-layer interferometry measurements were performed with an Octet HTX instrument (Fortébio) using AR2G biosensors. Data analyses were performed using FortéBio Data Analysis 9.0 software. Kinetic assays were performed at 25°C–30°C at a sample plate agitation speed of 1000 rpm. Sensors were first activated by immersion in a solution containing 20 mM of EDC and 10 mM of s-NHS. Then, 0.05 mg/mL of RBD antigen in 10 mM of sodium acetate pH6 was loaded for 600 seconds. After antigen loading, the biosensors were immersed in a solution of 1 M of ethanolamine pH8.5 to prevent nonspecific interactions. Antigen-loaded AR2G sensors were first dipped in PBS to establish a baseline time curve, and then immersed for 10 minutes in wells containing purified serum IgG at 3 different dilutions (3-5-8x). Following IgG association, dissociation was monitored for 600 seconds in PBS. Negative controls included ligand without IgG and IgG without ligand. Kinetic parameters were determined by global fitting of the association and dissociation phases of the binding curves according to a 1:1 binding model.
Statistics
Comparisons of antibody titers between groups were performed with the Kruskal-Wallis test with Dunn's multiple comparisons correction. Comparisons of antibody titers between age groups were performed with a 2-tailed Mann-Withney test. Correlations between antibody titers were analyzed with a 2-tailed Spearman test. Correlations between antibody titers and age were corrected with the Grubb's method to identify outliers; no outliers or a maximum of 1 outlier was identified per data set. A linear regression line was drawn, and a 2-tailed Spearman test was performed. Statistical analyses were performed using GraphPad Prism, version 9.3.1.
RESULTS
Children were recruited 3–5 months after a positive SARS-CoV-2 RT-PCR performed in a single hospital in Brussels between March and December 2020. Out of 136 children with a history of positive SARS-CoV-2 RT-PCR (Supplementary Table 1), 48 children were enrolled in the study (Table 1). Ten children were asymptomatic at the time of RT-PCR, 32 children had mild COVID-19 symptoms, and 6 were hospitalized, including 3 cases of MIS-C (Table 1). The interval between SARS-CoV-2 RT-PCR and analysis of antibody responses was similar in the 3 disease severity groups (Table 1). Male and female children had similar levels of antibodies for all measured parameters (data not shown).
Children Have Coordinated Antibody Responses to S, S1 Domain, and RBD but Not the S2 Domain of SARS-CoV-2
Levels of antibodies to SARS-CoV-2 S, S1 domain, RBD, and S2 domain were measured with a multiplex assay. Serum samples collected from healthy adults before the pandemic were used as technical negative controls. Overall, similar serum levels of IgG and IgA to SARS-CoV-2 antigens were detected in asymptomatic, mild, and hospitalized children, and these levels were higher than in adult negative controls (Figure 1A). High correlations were observed between serum titers of both IgG and IgA antibodies to S, S1, and RBD, whereas lower correlations were observed with antibodies to S2 (Figure 1B). Analysis of IgG and IgA subclasses indicated a similar pattern of correlations between antigen specificities (Supplementary Figure 1). Together, these data indicate that the antibody responses to RBD, S1 domain, and total S protein are coordinated, whereas a lower correlation is observed with the antibody response to the S2 domain, suggesting a differential regulation.
Figure 1.
Coordinated antibody response to SARS-CoV-2 spike protein, S1 domain, and RBD in children with asymptomatic or symptomatic COVID-19. A, Serum levels of IgG and IgA specific to SARS-CoV-2 S, S1 domain, RBD, or S2 domain were measured using a multiplex bead assay in 48 children with a positive SARS-CoV-2 RT-PCR. At the time of diagnosis, 10 children were asymptomatic (pink circles), 32 children had mild symptoms (violet circles), and 6 were hospitalized (3 non-MIS-C: purple circles; 3 MIS-C: purple squares). Sera from healthy adults collected before the COVID-19 pandemic were used as technical negative controls (n = 7, blue circles). Results are expressed as MFI. Data were analyzed using the Kruskal-Wallis test with Dunn's multiple comparisons correction. Bars indicate geometric mean antibody levels. B, Matrices indicating the correlations between titers of binding antibodies specific to the different target antigens. Results are 2-tailed r according to a colored scale. °: not significant. *P < .05; **P < .01; ***P < .001; ****P < .0001. Abbreviations: COVID-19, coronavirus disease 2019; MFI, mean fluorescence intensity; MIS-C, multisystem inflammatory syndrome; r, Spearman correlation coefficient; RBD, receptor binding domain; RT-PCR, reverse transcription polymerase chain reaction; S, spike protein; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
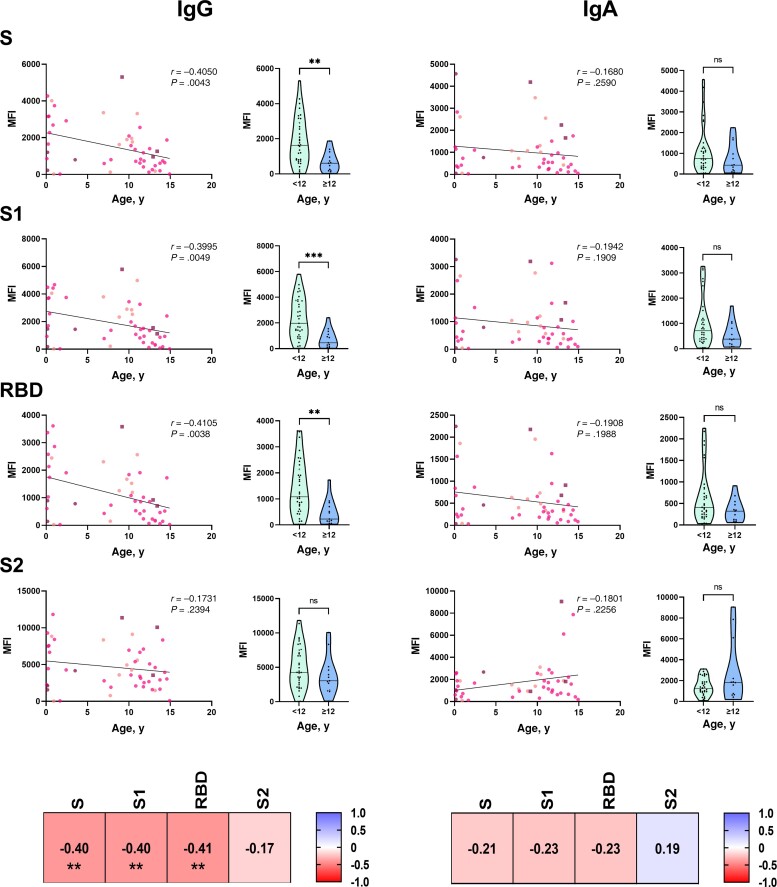
Younger Children Have Higher Antibody Responses to S, S1 Domain, and RBD but Not the S2 Domain of SARS-CoV-2 as Compared With Older Children
Relationships between antibody levels and age varied by SARS-CoV domain specificity (Figure 2). Unexpectedly, an inverse correlation was observed between the age of the children and the titers of S-, S1-, and RBD-specific IgG. Using an arbitrary cutoff of 12 years, a marked difference in antibody levels was observed between children <12 years of age and older children. In contrast, no significant difference in S2-specific IgG titers was observed between younger and older children. Higher levels of IgG subclasses (IgG1, IgG2, IgG3) specific to S, S1, and RBD were also detected in younger as compared with older children, whereas no significant difference was observed with S2-specific IgG subclasses (Supplementary Figure 2). Similar relationships were observed between age and the level of S-, S1-, RBD-, and S2-specific IgA, but these trends did not reach statistical significance (Figure 2). Together, these results indicate an unexpected capacity of younger children to produce higher levels of IgG to the S1 domain of SARS-CoV-2 S protein that does not extend to its S2 domain. Younger and older children had similar clinical characteristics (Supplementary Table 2).
Figure 2.
Younger age is associated with higher antibody response to spike protein, S1 domain, and RBD but not to S2 domain of SARS-CoV-2. Serum levels of IgG and IgA specific to SARS-CoV-2 total S, S1 domain, RBD, or S2 domain were measured using a multiplex bead assay in 48 children with a positive SARS-CoV-2 RT-PCR. Left panels: Each graph includes a regression line, a 2-tailed r, and a P value. At the time of diagnosis, 10 children were asymptomatic (pink circles), 32 children had mild symptoms (violet circles), and 6 were hospitalized (3 non-MIS-C: purple circles; 3 MIS-C: purple squares). Right panels: Violin plots indicate ranges of individual antibody levels and median antibody levels in children <12 years of age (n = 35, green plots) or ≥12 years of age (n = 12, blue plots). Binding antibody levels are expressed as MFI. Data were analyzed using the 2-tailed Mann-Whitney test. Correlation matrices indicate 2-tailed Spearman correlation coefficients and P values. **P < .01; ***P < .001. Abbreviations: Ig, immunoglobulin; MFI, mean fluorescence intensity; MIS-C, multisystem inflammatory syndrome; ns, nonsignificant; r, Spearman correlation coefficient; RBD, receptor binding domain; RT-PCR, reverse transcription polymerase chain reaction; S, spike protein; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Serum Levels of Antibodies to HCoVs Increase With Age in Children
In order to explore the potential role of back-boosting of immunity to HCoVs in the antibody response to SARS-CoV-2 infection, serum levels of IgG and IgA to HCoVs were measured. Similar levels of IgG and IgA specific to the S protein of beta (OC43 and HKU1) and alpha HCoVs (NL63 and 229E) were detected in the 3 disease severity groups (Supplementary Figure 3A). These levels were similar to those measured in sera collected from healthy adults before the pandemic. The relationship between age and the levels of HCoV-specific antibodies was then examined. Positive correlations were observed between age and serum levels of IgG and IgA to the S protein of beta and alpha HCoVs (Figure 3). Children age <12 years had lower levels of HCoV-specific IgG and IgA, although this difference only reached significance for alpha HCoVs. As expected, lower or no correlations were observed between the levels of antibodies to HCoVs and to the S, S1, and RBD domains of SARS-CoV-2 (Supplementary Figure 3B). In contrast, serum levels of IgA to beta HCoVs were correlated with antibodies to the S2 domain of SARS-CoV-2. A similar trend was observed between the level of beta HCoV and S2-specific IgG, but this trend did not reach statistical significance (Supplementary Figure 3B).
Figure 3.
Older age is associated with higher levels of antibodies to HCoVs. Serum levels of IgG and IgA specific to the S protein of beta HCoVs (OC43 and HKU1) and alpha HCoVs (NL63 and 229E) were measured using a multiplex bead assay in 48 children with a positive SARS-CoV-2 RT-PCR. Left panels: Each graph includes a regression line, a 2-tailed r, and a P value. At the time of diagnosis, 10 children were asymptomatic (pink circles), 32 children had mild symptoms (violet circles), and 6 were hospitalized (3 non-MIS-C: purple circles; 3 MIS-C: purple squares). Right panels: Violin plots indicate ranges of individual antibody levels and median antibody levels in children <12 years of age (n = 35, green plots) or ≥12 years of age (n = 12, blue plots). Binding antibody levels are expressed as MFI. Data were analyzed using the 2-tailed Mann-Whitney test. Correlation matrices indicate 2-tailed Spearman correlation coefficients and P values. *P < .05; **P < .01; ***P < .001; ****P < .0001. Abbreviations: HCoVs, common cold coronaviruses; MFI, mean fluorescence intensity; MIS-C, multisystem inflammatory syndrome; ns, not significant; r, Spearman correlation coefficient; RT-PCR, reverse transcription polymerase chain reaction; S, spike protein; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Younger Children Have Higher Serum Levels of SARS-CoV-2-Neutralizing and Complement-Activating Antibodies as Compared With Older Children
In order to explore the functional implications of the relationship between age and binding antibody levels, serum levels of neutralizing and complement-activating antibodies (ADCD) were measured. Higher levels of neutralizing antibodies to the Wuhan strain were detected in younger as compared with older children. These higher levels of neutralizing antibodies were associated with higher avidity of RBD IgG in younger children (Figure 4). No detectable neutralizing antibodies to the Omicron BA.1 variant were detected in younger or older children (Supplementary Figure 4). An inverse correlation with age was also observed with S, S1, and RBD-specific ADCD. In contrast, no correlation was observed between age and S2-specific ADCD, in line with binding antibody data. The levels of neutralizing antibodies, antibody avidity, and ADCD were highly correlated with the levels of binding antibodies to SARS-CoV-2 S, S1, and RBD, whereas lower correlations were detected with the levels of antibodies to the S2 domain, suggesting a dominant role of S1 epitopes not only in the neutralizing antibody responses but also in the ADCD response to SARS-CoV-2 (Supplementary Figure 4). Low or no correlations were observed between HCoV-specific antibodies and SARS-CoV-2-neutralizing antibodies or ADCD, indicating a limited role for cross-reactive antibodies in the functional antibody response to SARS-CoV-2 in children (Supplementary Figure 4). These data demonstrate that young children have a unique capacity to develop robust neutralizing and Fc-dependent functional antibody responses to SARS-CoV-2 and that this capacity does not correlate with their level of immunity to HCoVs.
Figure 4.
Younger age is associated with higher functional antibody responses to spike protein, S1 domain, and RBD but not S2 domain of SARS-CoV-2. Serum titers (IU/mL) of nAb SARS-CoV-2 Wuhan strain were measured using a microneutralization assay. Serum levels of SARS-CoV-2 total S, S1 domain, RBD, or S2 domain-specific ADCDs (MFI) were measured using a multiplex bead assay in 48 children with a positive SARS-CoV-2 RT-PCR. Avidity of RBD-specific IgG was measured by biolayer interferometry. Left panels: Each graph includes a regression line, a 2-tailed r, and a P value. At the time of diagnosis, 10 children were asymptomatic (pink circles), 32 children had mild symptoms (violet circles), and 6 were hospitalized (3 non-MIS-C: purple circles; 3 MIS-C: purple squares). Right panels: Violin plots indicate ranges of individual antibody levels and median antibody levels in children <12 years of age (n = 35, green plots) or ≥12 years of age (n = 12, blue plots). Functional antibody levels are expressed as MFI. Data were analyzed using the 2-tailed Mann-Whitney test. Correlation matrices indicate 2-tailed Spearman correlation coefficients and P values. *P < .05; **P < .01. Abbreviations: ADCDs, antibodies activating complement; Ig, immunoglobulin; MFI, mean fluorescence intensity; MIS-C, multisystem inflammatory syndrome; nAb, neutralizing antibody; ns, not significant; r, Spearman correlation coefficient; RBD, receptor binding domain; RT-PCR, reverse transcription polymerase chain reaction; S, spike protein; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
DISCUSSION
This study demonstrates an unexpected capacity of young children to develop effector antibody responses to SARS-CoV-2 infection. In contrast to adults, the magnitude of the antibody response to SARS-CoV-2 was similar across diverse clinical presentations of COVID-19 in children, confirming previous reports [23, 24].
Dowell at al. recently proposed that the higher levels of SARS-CoV-2 antibodies observed in children as compared with adults result from the back-boosting of a higher preexisting immunity to beta HCoVs [19, 20]. This model is not supported by our results showing that antibody levels to beta HCoVs follow an inverse relationship with age as compared with antibody levels to SARS-CoV-2, with higher levels in older as compared with younger children, most likely because of repeated exposures [14]. Our detailed analysis of the antibody response to individual domains of the S protein provides important insight into the role of cross-reactive immunity to HCoVs in the antibody response to SARS-CoV-2 infection in children. We observed that the levels of antibodies to the RBD and S1 domain of the S protein were highly coordinated and decreased with age, whereas the levels of antibodies to the S2 domain of the S protein were not correlated with age nor with the RBD or S1 domain. In contrast, levels of antibodies to the S2 domain were correlated with antibodies to beta HCoVs, in agreement with the high homology of this domain of SARS-CoV-2 with endemic coronaviruses. These data support a model involving independent regulation of the S1 domain of SARS-CoV-2, correlating with younger age, and of the S2 domain of SARS-CoV-2, correlating with older age and with immunity to beta HCoVs, in children. As our study did not include an analysis of samples collected before or during the acute phase of SARS-CoV-2 infection, back-boosting of antibodies to HCoVs could not be assessed. Importantly, antibody levels to the RBD and S1 domain, and not the S2 domain, correlated with antibodies to S proteins, suggesting that the S1 domain is the strongest determinant of antibody response to the total S protein in children. As a result, levels of both neutralizing and IgG Fc-dependent complement-activating antibodies correlated with levels of antibodies to the S protein and with younger age. Collectively, these data indicate that younger age is associated with higher effector antibody responses to SARS-CoV-2 and that this relationship cannot be explained by immunity to HCoVs. Since the submission of our report, 3 studies have been published indicating higher levels of SARS-CoV-2-binding and -neutralizing antibodies in younger as compared with older children, indicating the reproducibility of this observation across populations [25–27].
The mechanism that could underlie the inverse association between child age and levels of SARS-CoV-2 antibodies is unclear. The robust innate immune response detected early during the course of SARS-CoV-2 infection in children is likely to play a role [5, 13, 14, 16]. Trained immunity induced by common infections or vaccinations may contribute too [14]. Our observation that avidity of RBD-specific IgG inversely correlated with age of children suggests that younger children have a unique capacity to develop effective germinal center reactions in response to SARS-CoV-2 infection. Further studies are needed to assess whether the higher levels of high-avidity antibodies observed in younger children are associated with a different breadth of responses as compared with older children and adults. Our observations add to the evidence that adaptive immune responses are not defective in young children but follow different rules than in older children or in adults [11].
As younger children present milder COVID-19 than older children, the magnitude of antibody responses to SARS-CoV-2 could be an important factor contributing to the control of viral replication in this age group. Young children also have milder symptoms following SARS-CoV or MERS-CoV infection as compared with adults, but the impact of age on the antibody response to these pathogens is unknown [28]. Our results have important potential implications for herd immunity and for the role of children in the transmission of SARS-CoV-2. The persistence of high levels of antibodies 3–5 months after asymptomatic or mild infection in young children suggests that they may have a reduced risk of reinfection and transmission as compared with older children. Children had lower levels of neutralizing antibodies to the Omicron BA.1 variants as compared with the Wuhan strain, as observed in adults [29]. This suggests a limited ability to control reinfection with Omicron variants. Follow-up studies of natural immunity following infection with current variants of concern are needed to asses the role of children in herd immunity. As SARS-CoV-2 becomes endemic, the capacity of young children to develop potent antibody responses to this virus could provide an important contribution to herd immunity. Prevention of transmission has been proposed as an important argument in favor of COVID-19 vaccination in children [30]. Studies have suggested an impact of age on the antibody response to COVID-19 vaccination in SARS-CoV-2-naïve children, with either higher or lower responses detetected in younger as compared with older children, depending on the vaccine [31–33]. A comparison of natural vs vaccine-induced immunity in children will be important to further guide immunization strategies in this age group. On the other hand, children previously infected with SARS-CoV-2 may acquire potent hybrid immunity following vaccination, as observed in adults [34]. The limitations of this study include the relatively limited sample size and the assessment of SARS-CoV-2 immunity at a single time point following infection, preventing the analysis of the persistance of antibody levels.
In conclusion, this study demonstrates a unique capacity of young children to develop high effector antibody responses to SARS-CoV-2 infection, independent of their level of immunity to HCoVs. A high level of acquired immunity in children is likely to be an important factor in the control of the pandemic at the level of the population.
Supplementary Material
Acknowledgments
We thank the parents for consenting to the participation of their children in the study.
Financial support. This work was supported by grants from the territorial development agency of Wallonia “IDETA” provided by the Association Vésale, from the Fund for Scientific Research, F.R.S.-FNRS, from the Univerité Libre de Bruxelles, and by the Research Foundation Flanders (FWO G0G4220N), Belgium. A.M. is Research Director at the F.R.S.-FNRS, Belgium.
Contributor Information
Lisa Tomasi, Pediatric Department, Saint-Pierre Hospital, Brussels, Belgium.
Anais Thiriard, Institute for Medical Immunology, and ULB-Center for Research in Immunology, Université Libre de Bruxelles, Charleroi, Belgium.
Leo Heyndrickx, Virology Unit, Department of Biomedical Sciences, Institute of Tropical Medicine Antwerp, Antwerp, Belgium.
Daphnée Georges, Institute for Medical Immunology, and ULB-Center for Research in Immunology, Université Libre de Bruxelles, Charleroi, Belgium; Laboratory of Enzymology and Protein Folding, Centre for Protein Engineering, InBioS, University of Liège, Liège, Belgium.
Sigi Van den Wijngaert, Laboratoire Hospitalier Universitaire de Bruxelles (LHUB), Brussels, Belgium.
Véronique Olislagers, Institute for Medical Immunology, and ULB-Center for Research in Immunology, Université Libre de Bruxelles, Charleroi, Belgium.
Shilpee Sharma, Institute for Medical Immunology, and ULB-Center for Research in Immunology, Université Libre de Bruxelles, Charleroi, Belgium.
André Matagne, Laboratory of Enzymology and Protein Folding, Centre for Protein Engineering, InBioS, University of Liège, Liège, Belgium.
Margaret E Ackerman, Thayer School of Engineering, Dartmouth College, Hanover, New Hampshire, USA.
Kevin K Ariën, Virology Unit, Department of Biomedical Sciences, Institute of Tropical Medicine Antwerp, Antwerp, Belgium; Department of Biomedical Sciences, University of Antwerp, Antwerp, Belgium.
Tessa Goetghebuer, Pediatric Department, Saint-Pierre Hospital, Brussels, Belgium; Institute for Medical Immunology, and ULB-Center for Research in Immunology, Université Libre de Bruxelles, Charleroi, Belgium.
Arnaud Marchant, Institute for Medical Immunology, and ULB-Center for Research in Immunology, Université Libre de Bruxelles, Charleroi, Belgium.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
References
- 1. Preston LE, Chevinsky JR, Kompaniyets L, et al. Characteristics and disease severity of US children and adolescents diagnosed with COVID-19. JAMA Netw Open 2021; 4:e215298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brodin P. Why is COVID-19 so mild in children? Acta Paediatr 2020; 109:1082–3. [DOI] [PubMed] [Google Scholar]
- 3. Ludvigsson JF. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr 2020; 109:1088–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Blanchard-Rohner G, Didierlaurent A, Tilmanne A, et al. Pediatric COVID-19: immunopathogenesis, transmission and prevention. Vaccines (Basel) 2021; 9:1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brodin P. SARS-CoV-2 infections in children: understanding diverse outcomes. Immunity 2022; 55:201–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. World Health Organization . COVID-19 Disease in Children and Adolescents—Scientific Brief. World Health Organization; 2021. [Google Scholar]
- 7. MacLennan CA, Saul A. Vaccines against poverty. Proc Natl Acad Sci U S A 2014; 111:12307–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kollmann TR, Kampmann B, Mazmanian SK, et al. Protecting the newborn and young infant from infectious diseases: lessons from immune ontogeny. Immunity 2017; 46:350–63. [DOI] [PubMed] [Google Scholar]
- 9. Heath PT, Culley FJ, Jones CE, et al. Group B Streptococcus and respiratory syncytial virus immunisation during pregnancy: a landscape analysis. Lancet Infect Dis 2017; 17:e223–34. [DOI] [PubMed] [Google Scholar]
- 10. Abu-Raya B, Edwards KM, Scheifele DW, et al. Pertussis and influenza immunisation during pregnancy: a landscape review. Lancet Infect Dis 2017; 17:e209–22. [DOI] [PubMed] [Google Scholar]
- 11. Kollmann TR, Marchant A, Way SS. Vaccination strategies to enhance immunity in neonates. Science 2020;368:612–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schultze JL, Aschenbrenner AC. COVID-19 and the human innate immune system. Cell 2021; 184:1671–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vono M, Huttner A, Lemeille S, et al. Robust innate responses to SARS-CoV-2 in children resolve faster than in adults without compromising adaptive immunity. Cell Rep 2021; 37:109773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zimmermann P, Curtis N. Why does the severity of COVID-19 differ with age? Pediatric Infect Dis J 2022; 41:e36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Moss P. The T cell immune response against SARS-CoV-2. Nat Immunol 2022; 23:186–93. [DOI] [PubMed] [Google Scholar]
- 16. Chou J, Thomas PG, Randolph AG. Immunology of SARS-CoV-2 infection in children. Nat Immunol 2022; 23:177–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Garrido C, Hurst JH, Lorang CG, et al. Asymptomatic or mild symptomatic SARS-CoV-2 infection elicits durable neutralizing antibody responses in children and adolescents. JCI Insight 2021; 6:e150909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yang HS, Costa V, Racine-Brzostek SE, et al. Association of age with SARS-CoV-2 antibody response. JAMA Netw Open 2021; 4:e214302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dowell AC, Butler MS, Jinks E, et al. Children develop robust and sustained cross-reactive spike-specific immune responses to SARS-CoV-2 infection. Nat Immunol 2022; 23:40–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lavinder JJ, Ippolito GC. Boosted immunity to the common cold might protect children from COVID-19. Nat Immunol 2022; 23:8–10. [DOI] [PubMed] [Google Scholar]
- 21. Crowley AR, Natarajan H, Hederman AP, et al. Boosting of cross-reactive antibodies to endemic coronaviruses by SARS-CoV-2 infection but not vaccination with stabilized spike. Elife 2022; 11:e75228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pannus P, Neven KY, De Craeye S, et al. Poor antibody response to BioNTech/Pfizer COVID-19 vaccination in SARS-CoV-2 naïve residents of nursing homes. Clin Infect Dis 2022; 75:e695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Weisberg SP, Connors TJ, Zhu Y, et al. Distinct antibody responses to SARS-CoV-2 in children and adults across the COVID-19 clinical spectrum. Nat Immunol 2021; 22:25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Renk H, Dulovic A, Seidel A, et al. Robust and durable serological response following pediatric SARS-CoV-2 infection. Nat Commun 2022; 13:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ruedas-López A, Berzosa-Sánchez A, Illán-Ramos M, et al. Longitudinal survey of humoral and cellular response to SARS-CoV-2 infection in children. Microbiol Res 2022; 264:127145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Han MS, Um J, Lee EJ, et al. Antibody responses to SARS-CoV-2 in children with COVID-19. J Pediatric Infect Dis Soc 2022; 11:267–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yang HS, Costa V, Racine-Brzostek SE, et al. Association of age with SARS-CoV-2 antibody response. JAMA Netw Open 2021; 4:e214302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zimmermann P, Curtis N. Coronavirus infections in children including COVID-19. Pediatric Infect Dis J 2020; 39:355–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ariën KK, Heyndrickx L, Michiels J, et al. Three doses of BNT162b2 vaccine confer neutralising antibody capacity against the SARS-CoV-2 Omicron variant. NPJ Vaccines 2022; 7:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zimmermann P, Pittet LF, Finn A, et al. Should children be vaccinated against COVID-19? Arch Dis Child 2022; 107:e1. [DOI] [PubMed] [Google Scholar]
- 31. Zhu F, Jin P, Zhu T, et al. Safety and immunogenicity of a recombinant adenovirus type-5-vectored COVID-19 vaccine with a homologous prime-boost regimen in healthy participants aged 6 years and above: a randomised, double-blind, placebo-controlled, phase 2b trial. Clin Infect Dis 2022; 75:e783–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Frenck RW, Klein NP, Kitchin N, et al. Safety, immunogenicity, and efficacy of the BNT162b2 COVID-19 vaccine in adolescents. New Engl J Medicine 2021; 385:239–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Xia S, Zhang Y, Wang Y, et al. Safety and immunogenicity of an inactivated COVID-19 vaccine, BBIBP-CorV, in people younger than 18 years: a randomised, double-blind, controlled, phase 1/2 trial. Lancet Infect Dis 2022; 22:196–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Crotty S. Hybrid immunity. Science 2021; 372:1392–3. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.