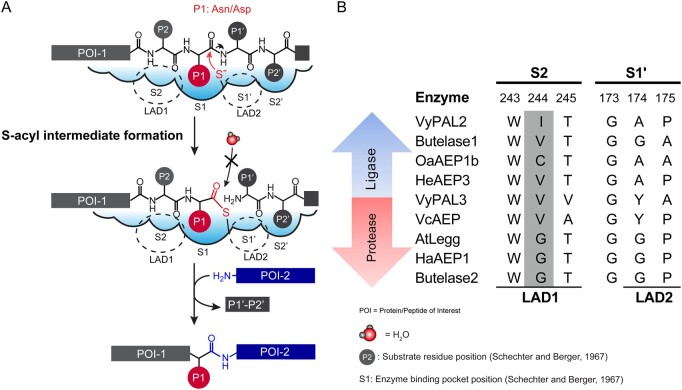
Figure 1.
Mechanism of ligation by PALs, and amino-acid composition of the S2 and S1′ pockets. A, Two steps are necessary for the reaction. First, the substrate peptide binds to the active site, with the P1 Asx bound into the S1 pocket. The catalytic cysteine performs a nucleophilic attack that breaks the peptide bond between the P1 and P1′ residues; as a result, a thioester intermediate is formed. The second step is the resolution of the intermediate by an incoming nucleophile (a water molecule in the case of hydrolysis and the amine group of a peptide in the case of a ligation reaction). Substrate P4–P1 and P1′–P2′ binding occurs via interactions with specific residues in the S1–S4 and S1′–S2′ pockets. Nucleophilic attack by the catalytic cysteine on the P1 residue carbonyl carbon atom leads to the formation of the acyl-enzyme intermediate. Nucleophilic attack by a water molecule is inhibited to favour nucleophilic attack by the amine group of the incoming peptide. B, Nature of residues in the S2 (LAD 1 region) and S1′ (LAD2 region) pockets characteristic of ligases and proteases. The numbers above the sequence alignment refer to residue numbers in VyPAL2. Residues in the Gatekeeper position are shaded in gray. Abbreviations for amino acids are as follows: A: Ala, C: Cys, D: Asp, E: Glu, F: Phe, G: Gly, H: His, I: Ile, K: Lys, L: Leu, M: Met, N: Asn, P: Pro, Q: Gln, R: Arg, S; Ser, Snn: Asi (Aspartimide), T: Thr, V: Val, W: Trp, Y: Tyr.