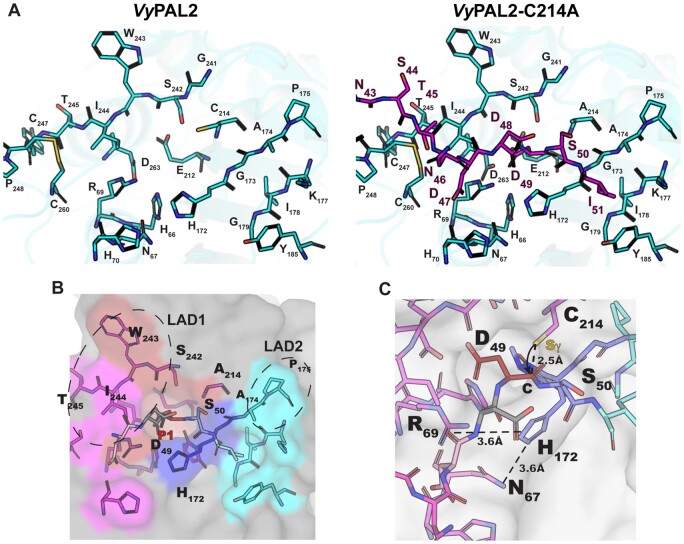
Figure 5.
Peptide-binding groove and substrate recognition by VyPAL2. A, The left part shows the peptide binding groove of the ligase, as derived from the crystal structure of VyPAL2 (Supplemental Table S2). The right part is derived from the structure of VyPAL2-C214A (Supplemental Table S2, form I) bound to the peptide displayed in magenta. B, Residues lining the substrate binding pockets are depicted as sticks. The VyPAL2 core domain is represented as a gray surface. The S1 pocket is red, the S3 pocket is magenta, the S1′ pocket is blue, the S2′ pocket is cyan. C, The active site residues His172 and C214 (mutated here to Ala) are on opposite sides of the peptide substrate, and adding back a sulfhydryl group at Ala214 to the model shows that the distance from the scissile carbon is consistent with an inline attack of the carbon atom of the carbonyl group as displayed.