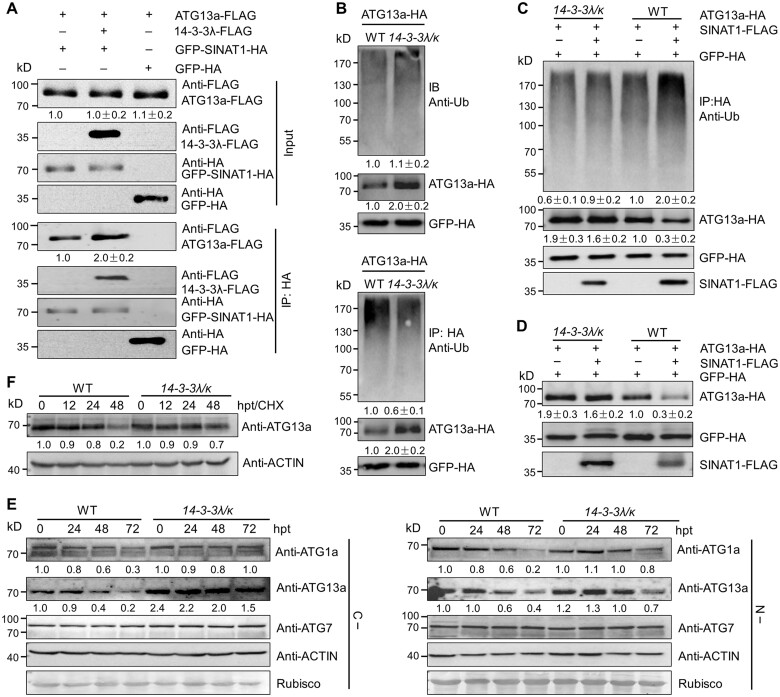
Figure 6.
14-3-3λ and 14-3-3κ regulate the SINAT1-mediated ubiquitination and degradation of ATG13a. A, 14-3-3 proteins increase the interaction of SINAT1 and ATG13a. In vivo Co-IP assay showing the interaction between ATG13a-FLAG and GFP-SINAT1-HA in the presence of 14-3-3λ-FLAG. Constructs encoding ATG13a-FLAG and GFP-SINAT1-HA with or without 14-3-3λ-FLAG were transiently co-transfected into Col-0 protoplasts (Col-0) and incubated under light conditions for 16 h before immunoprecipitation with HA affinity agarose beads. GFP-HA was co-transfected with ATG13a-FLAG as negative control. Numbers on the left indicate the molecular weight (kDa) of each band. Relative intensity of the immunoprecipitated ATG13a-FLAG normalized to the GFP-SINAT1-HA is shown below. B, Ubiquitination of ATG13a in the 14-3-3λ 14-3-3k double mutant. ATG13a-HA was transiently transfected into Arabidopsis protoplasts isolated from 4-week-old WT and 14-3-3λ 14-3-3k mutant plants, and the ubiquitination of ATG13a was detected by immunoprecipitation and immunoblot analysis. Proteins were extracted after a 16-h incubation under constant light conditions, followed by the addition of HA affinity agarose beads. The proteins were detected with anti-HA and anti-Ub antibodies. Relative intensity of each protein normalized to the GFP-HA is shown below. The relative intensity of each protein is the mean ± sd calculated from three independent experiments. C, SINAT1-mediated ubiquitination of ATG13a is attenuated in the 14-3-3λ 14-3-3k double mutant. ATG13a-HA and SINAT1-FLAG constructs were transiently co-transfected into Arabidopsis protoplasts prepared from WT or 14-3-3λ 14-3-3k plants and incubated for 16 h under constant light conditions. ATG13a-HA ubiquitination was determined with anti-Ub and anti-HA antibodies. Relative intensity of each protein normalized to the GFP-HA is shown below. The relative intensity of each protein is the mean ± sd calculated from three independent experiments. D, SINAT1-associated degradation of ATG13a is dependent on 14-3-3λ and 14-3-3κ. ATG13a-HA and SINAT1-FLAG constructs were transiently co-transfected into Arabidopsis protoplasts prepared from WT and 14-3-3λ 14-3-3k double mutant plants and incubated for 16 h under continuous light conditions. ATG13a-HA stability was determined with anti-HA antibodies. Relative intensity of each protein normalized to GFP-HA is shown below. The relative intensity of each protein is the mean ± sd calculated from three independent experiments. E, ATG1a, ATG13a, and ATG7 protein abundance in 14-3-3λ 14-3-3k double mutant seedlings. One-week-old WT and 14-3-3λ 14-3-3k mutant seedlings were subjected to C (C–; upper images) or N starvation (N–; lower images) treatment for 24, 48, or 72 h. The blots were probed with anti-ATG1a, anti-ATG13a, and anti-ATG7 specific antibodies. Relative intensity of each protein normalized to the loading control is shown below. F, Stability of ATG13a protein in the 14-3-3λ 14-3-3k double mutant and WT seedlings upon CHX treatment. One-week-old WT and 14-3-3λ 14-3-3k double mutant seedlings were treated with 0.5-mM CHX for 0, 12, 24, or 48 h. Specific anti-ATG13a antibodies were used for immunoblot analysis. Relative intensity of each protein normalized to the loading control is shown below. Numbers on the left indicate the molecular weight (kDa) of each band. GFP-HA was used as control for transfection efficiency. Anti-ACTIN antibodies and Ponceau S-stained membranes are shown below the blots to indicate the amount of protein loaded per lane.