Extended Data Fig. 5|. Biochemical characterization of the EZH2-targeting PROTAC degrader, MS177.
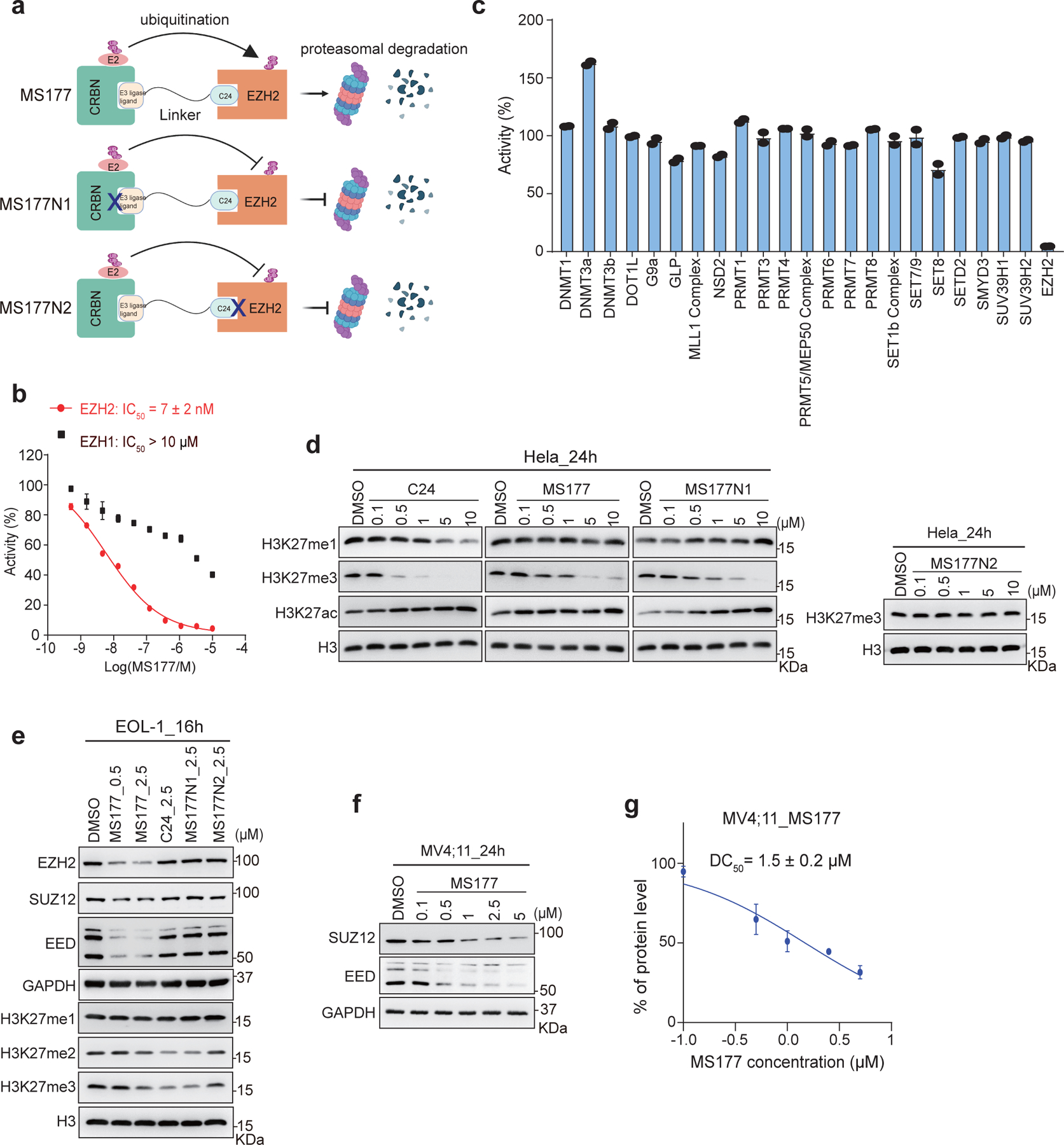
(a) Scheme showing the expected effect by MS177, MS177N1 (which contains a moiety that does not bind CRBN; indicated by a cross-mark) and MS177N2 (which contains a moiety that does not bind EZH2).
(b) A radioactive methyltransferase assay (3H-labeled S-Adenosyl methionine [SAM] as methyl donor) showing that MS177 exhibits a high inhibition potency for EZH2 and a high selectivity for EZH2 over EZH1. X-axis and y-axis show the used concentration of MS177 (in Log scale) and the rate of inhibition (treatment versus mock), respectively (n=3; mean ± SD). IC50, half maximal inhibitory concentration.
(c) Selectivity of MS177 (10 μM, relative to mock) against a panel of 23 different lysine, arginine or DNA methyltransferases in radioactive methyltransferase assays (n=3; mean ± SD).
(d) Immunoblotting of the indicated histone modification in Hela cells after a 24-hour treatment with different concentrations of C24, MS177, MS177N1 or MS177N2, in comparison to mock (DMSO).
(e) Immunoblotting of PRC2 subunits (GAPDH as a loading control) and global H3K27 methylation levels (H3 as a loading control) in EOL-1 cells post-treatment with DMSO, the indicated concentrations of MS177, or 2.5 μM of C24, MS177N1 or MS177N2 for 16 hours.
(f) Immunoblotting of PRC2 subunits (GAPDH as a loading control) in MV4;11 cells after a 24-hour treatment with the increasing concentration of MS177, relative to mock (DMSO).
(g) Measurement of half-maximal degradation concentration (DC50) value of MS177 in MV4;11 cells, based on EZH2 immunoblotting signals in the MS177-treated and mock-treated cells (n=2 independent experiments; mean ± SD; quantified with ImageJ).
