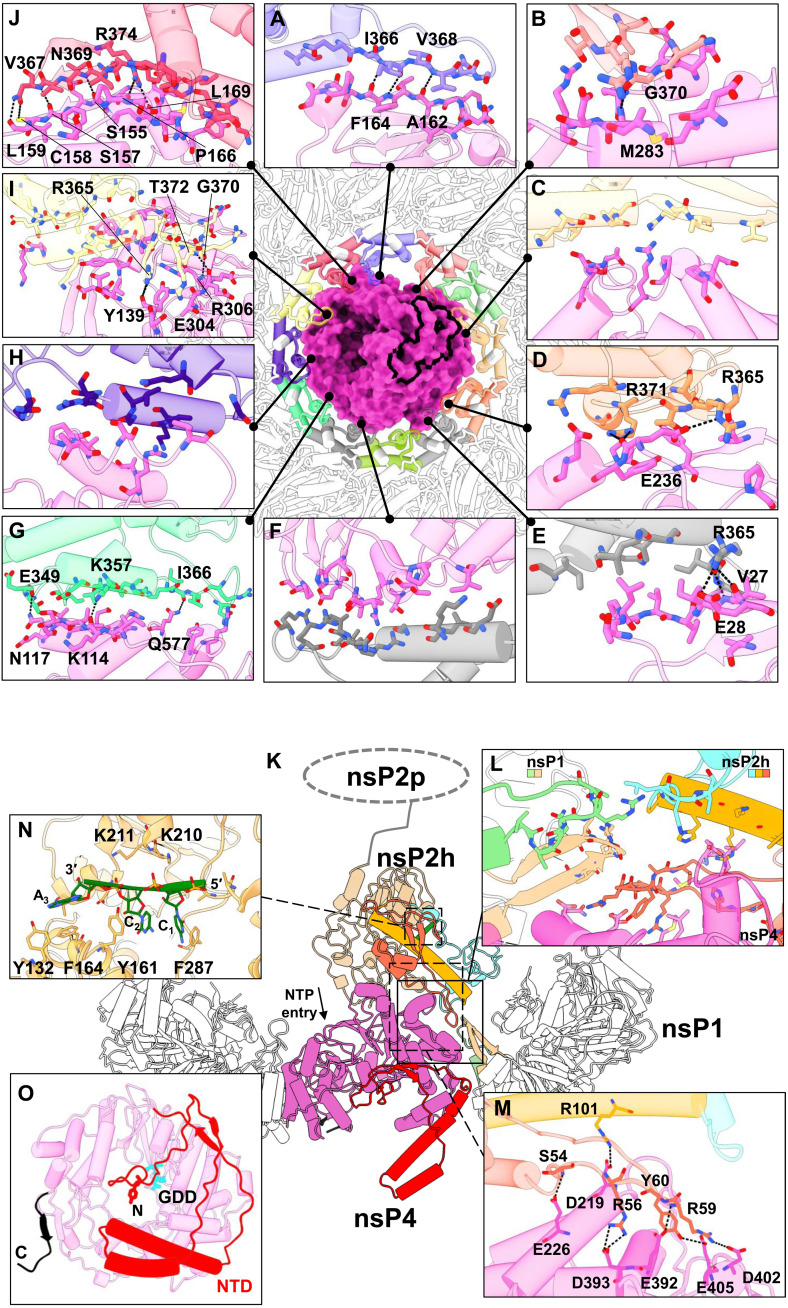
Fig. 2. The macromolecular architecture of the RC displays a multiple interface network.
(A to J) The interaction network of RC (made of nsP1 + 2 + 4 at the top view) is presented with each colored by each subunit chain for nsP1 dodecameric ring and nsP4 (surface), based on Fig. 1 chain coloring. For clarity of the interaction overview, nsP2 and its RNA ligand are hidden. Instead, the nsP2:nsP4 interface area is outlined with a thick black line. The hydrogen bonds (black dotted lines) between the individual chain of nsP1 and nsP4 are shown here in the interface blown-up view with each residue involved named and colored according to the chain. (K) The overall side-view impression of interfaces spanning across CHIKV RC (cartoon representations) where their three-way interactions between nsP1, nsP2, and nsP4 are shown in (L) a blown-up window (solid line and box). The nsP2h (amino acids 1 to 465) region is colored according to its subdomain: NTD in orange, STALK in gold, 1B in cyan, and RecA1-A2 in peru, while chains C and D of nsP1 are respectively colored in light green and tan. The unbuilt nsP2 protease (nsP2p; after amino acids 466) region is drawn here at the C terminus of nsP2h for visual guidance for (K). NTP entry site at the nsP4 motif D within the palm subdomain (magenta region) is annotated. (M) The hydrogen bonds (dotted lines) between the nsP2:nsP4 interface are listed on another blown-up window (dotted line and box). (N) The interacting residues from nsP2h and RNA (green) are labeled at a zoomed-in view (dotted line and box). (O) The bottom view of nsP4 showcases the spatial coordinates of its C terminus (C; black; amino acids 600 to 611) and N-terminal domain (NTD; red; amino acids 1 to 105) and the active site (named GDD; cyan stick).