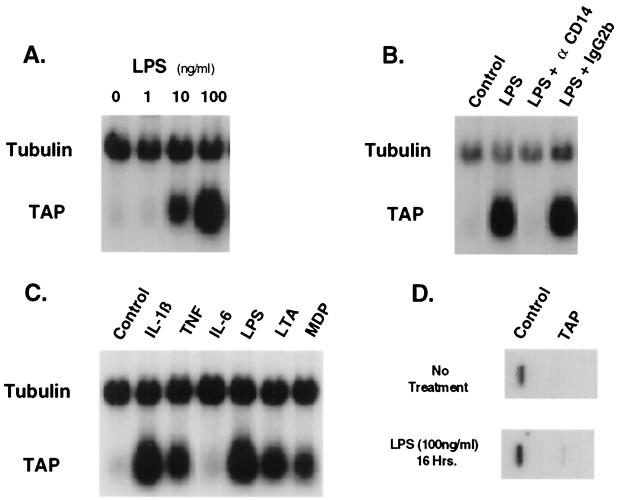
FIG. 1.
Induction of TAP mRNA levels in TEC by LPS and other inflammatory mediators. (A) Concentration dependence of LPS on TAP mRNA levels in bovine TEC. Total RNA was isolated from primary cultures of TEC (2 × 105 cells/culture) after incubation for 16 h with no additions (lane 1) or in the presence of purified LPS from Pseudomonas aeruginosa (1 ng/ml [lane 2], 10 ng/ml [lane 3], or 100 ng/ml [lane 4]). Probes for Northern blot analysis were TAP48a (TAP) and α-tubulin cDNA (Tubulin). (B) Effect of My4, an anti-CD14 blocking monoclonal antibody, on LPS-inducible changes in TAP mRNA levels. Cultures of TEC were incubated without LPS (lane 1) or with LPS (100 ng/ml) (lanes 2 to 4) for 16 h. The anti-CD14 (αCD14) mouse monoclonal antibody My4 (500 ng/ml) (lane 3) or an equivalent concentration of immunoglobulin G2b (IgG2b), an isotype-specific control antibody (lane 4), was coincubated as indicated. Northern blot analysis was as described for panel A. (C) Effects of inflammatory cytokines and bacterial products on TAP mRNA levels. RNA was isolated from TEC after incubation for 16 h with no additions (lane 1) or in the presence of TNF-α (100 ng/ml) (lane 2), IL-1β (100 ng/ml) (lane 3), IL-6 (500 U/ml) (lane 4), LPS (100 ng/ml) (lane 5), lipoteichoic acid (LTA) (10 μg/ml) (lane 6), or muramyl dipeptide (MDP) (100 μg/ml) (lane 7). Northern blot analysis was as described for panel A. (D) Nuclear run-on transcription of TAP in TEC. TEC were untreated (Control) or treated with bacterial LPS (100 ng/ml) for 16 h. Nuclei from 5 × 106 cells were harvested and used for nuclear run-on analysis as described in Materials and Methods. Nylon filter blots containing cDNA encoding TAP and tubulin were hybridized under high-stringency conditions with 32P-labeled primary RNA transcripts. Specific hybridization of RNA was assessed by autoradiography and quantitated by phosphorimager analysis.