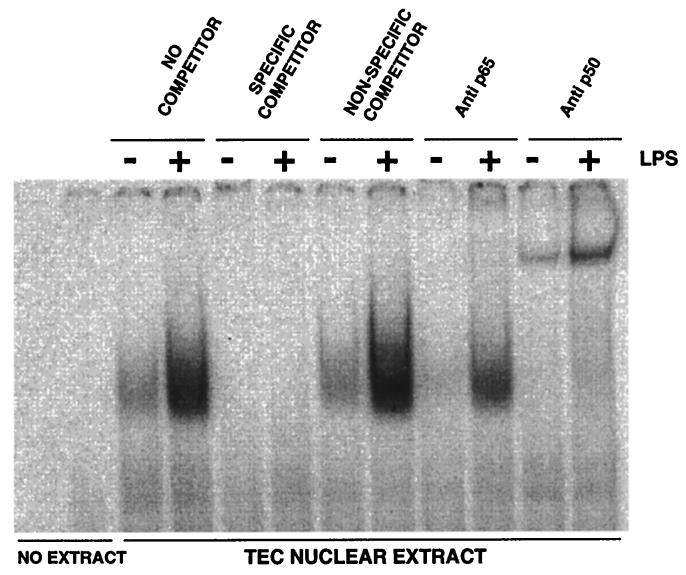
FIG. 3.
Mobility shift analysis of NF-κB in bovine TEC nuclear extracts. A 32P-labeled, double-stranded oligonucleotide probe containing the NF-κB sequence flanking the TAP gene (ds-TAP/NF32) was incubated with nuclear extracts from bovine TEC treated with LPS (100 ng/ml) or untreated. Specificity of binding is shown by competition with unlabeled specific (ds-TAP/NF32) and nonspecific (ds-TAP/NFmut32) double-stranded oligonucleotides, as described in Materials and Methods. The composition of the NF-κB complex was analyzed by including either anti-p50 or anti-p65 antibodies in the binding reaction. Inclusion of the anti-p50 antibody causes retarded migration of the binding complex, whereas anti-p65 antibody decreases the signal intensity of the binding complex.