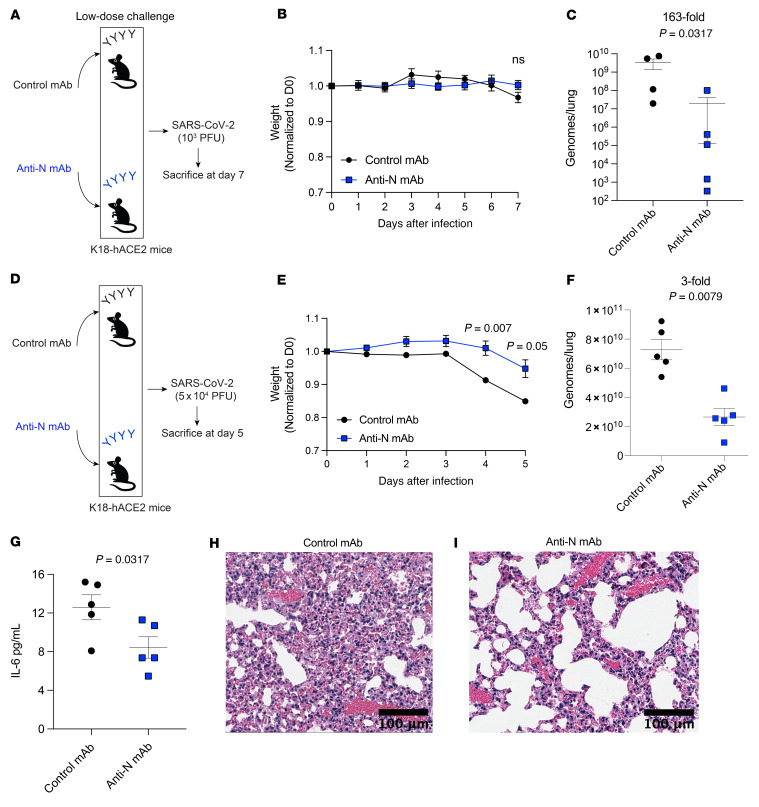
Figure 6. Nucleocapsid-specific mAb improves the control of SARS-CoV-2 infection.
(A) Experimental approach for evaluating viral control after treatment with a nucleocapsid-specific mAb during a low-dose viral challenge. mAbs (800 μg, IgG control or anti-N) were injected intraperitoneally into naive K18-hACE2 mice. On the following day, the K18-hACE2 mice were challenged intranasally with 103 PFU SARS-CoV-2. (B) Weight loss following infection. (C) Viral loads in lungs as determined by RT-qPCR. RNA was harvested from the lungs on post-infection day 7, and viral RNA was quantified. (D) Experimental approach for evaluating viral control after treatment with a nucleocapsid-specific mAb during a high-dose viral challenge. mAbs (800 μg, IgG control or anti-N) were injected intraperitoneally into naive K18-hACE2 mice. On the following day, the K18-hACE2 mice were challenged intranasally with 5 × 104 PFU SARS-CoV-2. (E) Weight loss following infection. (F) Viral loads in lungs as determined by RT-qPCR. RNA was harvested from the lungs on post-infection day 5, and viral RNA was quantified. (G) IL-6 levels in sera. (H and I) H&E-stained images of lung. Scale bars: 100 μm. Data in A–C are from low-dose viral challenges, and data in D–I are from high-dose viral challenges. Challenges were performed with a total of 4–5 mice per group in BSL-3 facilities. P values were calculated using a Mann-Whitney U test. Error bars represent the SEM.