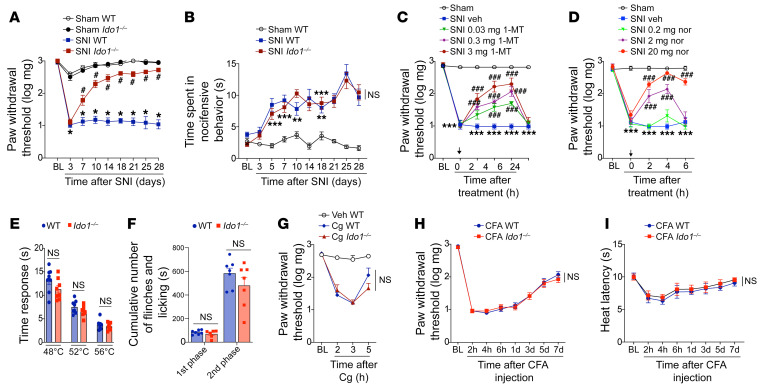
Figure 2. IDO1 is involved in the maintenance of neuropathic pain but has no role in nociceptive or inflammatory pain.
(A) Mechanical and (B) cold nociceptive responses were evaluated before and up to 28 days after SNI and sham surgeries in WT and Ido1–/– mice. (n = 5–9). (C and D) Mechanical nociceptive threshold was determined before and 14 days after SNI. Mice were treated intraperitoneally (i.p.) with vehicle or 1-methyl-DL-tryptophan (1-MT, 0.03–3 mg/mouse) or norharmane (nor, 0.2–20 mg/kg), and mechanical allodynia was measured up to 24 hours after treatment (n = 5). (E) The nociceptive thermal threshold was tested in naive WT and Ido1–/– mice at 48°C, 52°C, and 56°C using the hot-plate test (n = 8). (F) Formalin (1%) was used to produce overt pain-like behavior. Total duration (seconds) of nociceptive behaviors for 0 to 10 minutes (1st phase) and for 10 to 50 minutes (2nd phase) after formalin injection was evaluated in WT and Ido1–/– mice (n = 7). (G) Mechanical nociceptive threshold using von Frey test (n = 4–5) was evaluated in WT and Ido1–/– mice followed by intraplantar injection of carrageenan (Cg, 100 μg per paw) or vehicle (saline). (H and I) Mechanical and thermal (heat) nociceptive threshold using von Frey test (n = 7) and Hargreaves’ test, respectively, were evaluated in WT and Ido1–/– mice followed by intraplantar injection of CFA (10 μL per paw). Data are expressed as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001 versus sham or vehicle; #P < 0.05, ###P < 0.001 versus Ido1–/– mice by 2-way ANOVA with Bonferroni’s post hoc test (A–D and G–I) or unpaired, 2-tailed Student’s t test (E and F). NS, no statistical significance.