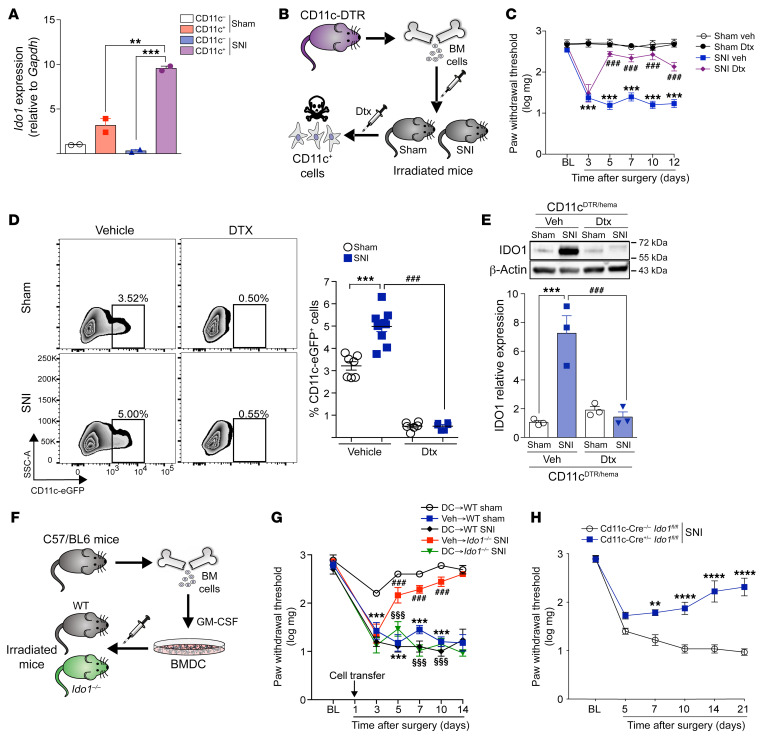
Figure 4. DC-expressed IDO1 contributes to the maintenance of neuropathic pain.
(A) Ido1 mRNA expression in CD11c+ or CD11c– cells isolated from the draining lymph nodes 14 days after sham or SNI surgery (n = 2 pooled from 5). (B) Representative scheme of chimeric CD11cDTR/hema mice establishment. (C) Mechanical nociceptive threshold was determined before and up to 12 days after sham or SNI surgery in chimeric CD11cDTR/hema mice treated with vehicle or diphtheria toxin (Dtx; 16 ng Dtx/g, i.p.) (n = 6). (D) Representative dot plots and quantification (percentage) of CD11c+ DCs in the draining lymph nodes harvested 14 days after sham or SNI surgery from chimeric CD11cDTR/hema mice treated with vehicle or Dtx (n = 6–10). (E) Western blotting analysis of IDO1 expression in the draining lymph nodes harvested 14 days after sham or SNI surgery from chimeric CD11cDTR/hema mice treated with vehicle or Dtx (n = 3). (F) Representative scheme of DC differentiation and transfer to Ido1–/– or WT mice. (G) Mechanical nociceptive threshold before (BL) and up to 14 days after sham or SNI in WT and Ido1–/– mice that received in vitro–differentiated DCs 1 day after surgeries (n = 5–6). (H) Mechanical nociceptive threshold was evaluated before and up to 21 days after SNI surgery in mice conditionally lacking Ido1 in CD11c+ cells (CD11c-Cre+/– Ido1fl/fl mice) or control littermates (CD11c-Cre–/– Ido1fl/fl) (n = 4–6). Data are expressed as mean ± SEM. **P < 0.01, ***P < 0.001, ****P < 0.0001 versus sham littermate control; ###P < 0.001 versus WT mice or treatment; §§§P < 0.001 versus BM Ido1–/– by 1-way ANOVA with Bonferroni’s post hoc test (A, D, and E) or 2-way ANOVA with Bonferroni’s post hoc test (C, G, and H).