FIGURE 1.
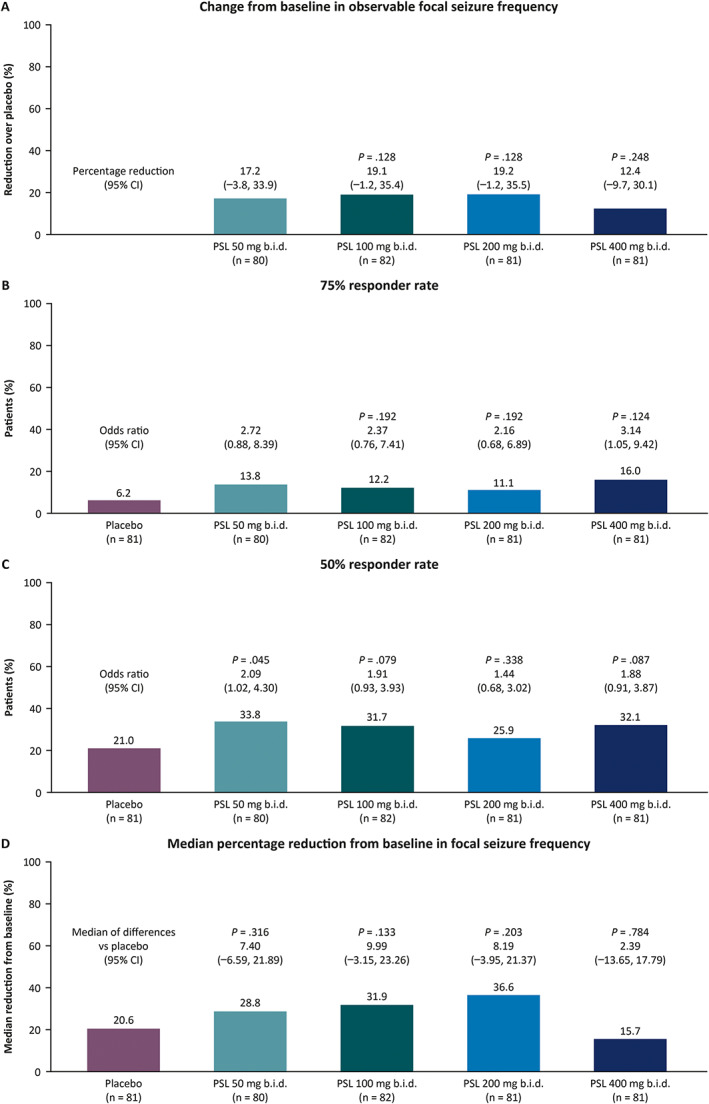
Efficacy outcomes of patients randomized to placebo or PSL by randomized dose group for observable focal seizures over the 12‐week maintenance period in the dose‐finding trial (EP0091; full analysis set): (A) change from baseline in log‐transformed observable focal seizure frequency as percentage reductions over placebo; (B) 75% responder rate; (C) 50% responder rate; (D) median percentage reduction from baseline in focal seizures per 28 days. For the primary outcomes (percentage reductions over placebo in observable focal seizure frequency and 75% responder rates), statistical comparison of the PSL 50 mg b.i.d. dose group to placebo was provided only if the other three higher doses were significant. ASM, antiseizure medication; b.i.d., twice daily; PSL, padsevonil.
