FIGURE 2.
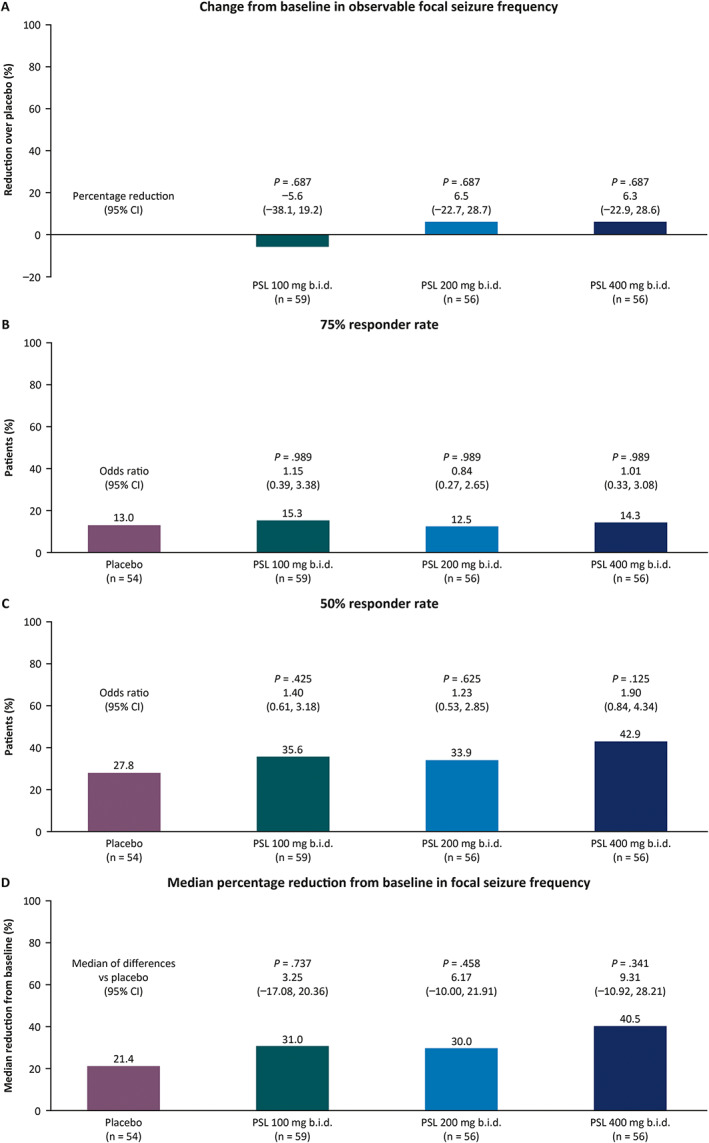
Efficacy outcomes of patients randomized to placebo or PSL by randomized dose group for observable focal seizures over the 12‐week maintenance period in the phase 3 efficacy trial (EP0092; full analysis set): (A) change from baseline in log‐transformed observable focal seizure frequency as percentage reductions over placebo; (B) 75% responder rate; (C) 50% responder rate; (D) median percentage reduction from baseline in focal seizures per 28 days. ASM, antiseizure medication; b.i.d., twice daily; PSL, padsevonil.
