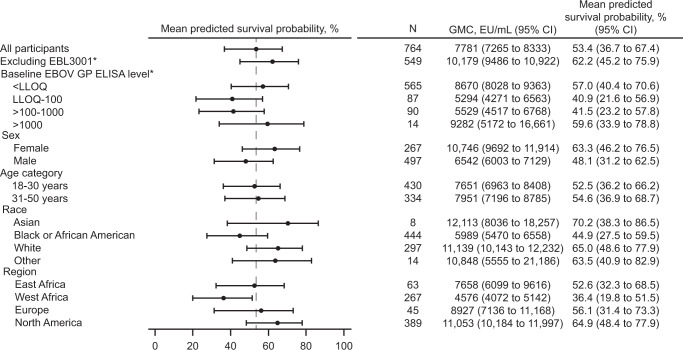
Fig. 1. Forest plot of mean predicted survival probability and 95% CI – prespecified subgroup analyses by baseline EBOV GP binding antibody concentration, sex, age category, race, and region; PPI analysis set.
This analysis was based on the pooled data of healthy adults (aged 18-50 years) vaccinated with Ad26.ZEBOV, MVA-BN-Filo in a 56-day interval in five clinical studies (EBL2001, EBL2002, EBL3001, EBL3002, and EBL3003) using a logistic regression model based on NHP data from the Ad26.ZEBOV, MVA-BN-Filo vaccine regimen in a 56-day interval. Mean predicted survival probability and the 95% bootstrapped CI are reported. CI confidence interval, EBOV Ebola virus, ELISA enzyme-linked immunosorbent assay, EU enzyme-linked immunosorbent assay units, GMC geometric mean concentration, GP glycoprotein, LLOQ lower limit of quantification (36.11 EU/mL), N number of participants with data, NHP non-human primate, PPI per-protocol immunogenicity, vertical dashed line = mean predicted survival probability from primary analysis including all participants. *The subgroup analyses excluding the participants from clinical study EBL3001 and the stratification by baseline EBOV GP ELISA level are described in more detail in the Supplementary Results.