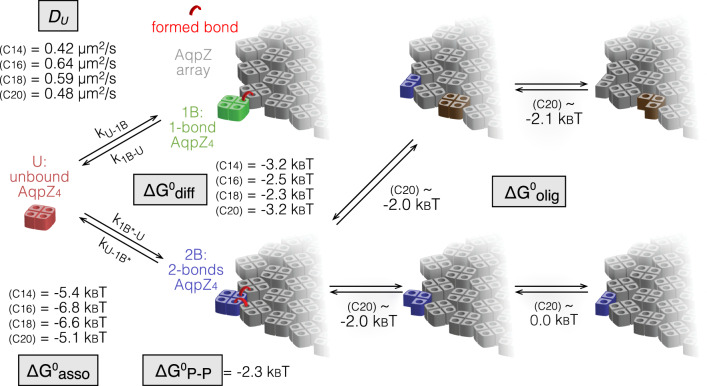
Fig. 5. AqpZ oligomerization and assembly.
The AqpZ-W14A oligomerization energetics was estimated based on observation statistics of non-tetrameric complexes. The protomer interaction ΔG0olig is weakest (~−2 kBT) in C20 lipids that match the hydrophobic thickness of the protomer interface. AqpZ diffusion DU is slowed in lipids with larger hydrophobic mismatch (thicker and thinner). Membrane-mediated membrane protein interaction ΔG0asso is most favorable (~−6.5 kBT) in lipids with thickness close to the hydrophobic thickness of the protein. Bond formation with two array-bound proteins, filling gaps in the 2D-plane, provides a maximum energy gain ΔG0diff in lipids with strong mismatch (~−3 kBT). The latter driving the assembly towards the formation of membrane protein arrays. The direct (not membrane-mediated) protein–protein interaction ΔG0P-P (~−2 kBT) stabilizes these interactions at very short distances.