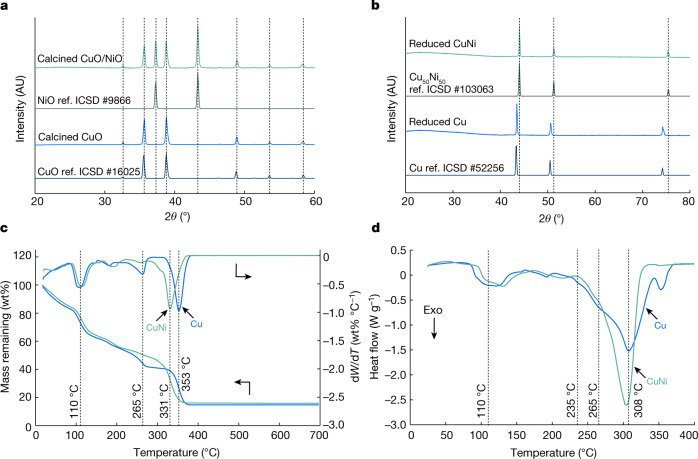
Fig. 3. Chemical characterization of hydrogel infusion produced metals and alloys.
a, XRD patterns of calcined gels: Cu(NO3)2 gel is converted to CuO and Cu(NO3)2/Ni(NO3)2 gel is converted to CuO/NiO. b, XRD patterns of oxides reduced to parent metals: CuO is converted to Cu and CuO/NiO is converted to a homogenous CuNi alloy, as evidenced by the single set of FCC reflections. c, TGA profiles of metal-ion-infused gels heated to 700 °C in air at 1 °C min−1 reveal rapid mass loss events reaching maxima at 353 °C for Cu and 331 °C for CuNi. d, DSC profiles of metal-ion-infused gels heated to 400 °C in air at 1 °C min−1 reveal exothermic (Exo) events with maximum heat flow at 308 °C for Cu and at 304 °C for CuNi. AU, arbitrary units. Ref. ICSD #, reference pattern from Inorganic Crystal Structure Database (see Methods).