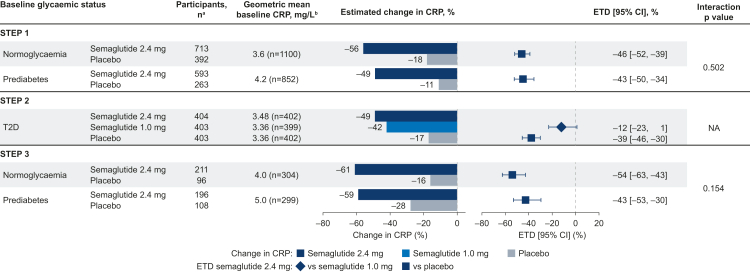
Fig. 3.
Change in CRP from baseline to week 68 by baseline glycaemic status. Estimated data for the treatment policy estimand (assessed the treatment effect in all participants, regardless of trial product discontinuation or use of other anti-obesity therapies), analysed using the full analysis set. Changes from baseline and ETDs are based on estimated ratios to baseline and estimated treatment ratios. They are presented as estimated percent changes and estimated relative percent difference between groups, respectively, converted using the formula (estimated ratio − 1) × 100. Data for the T2D population are based on the full analysis set in STEP 2 and therefore subgroup interactions were not assessed. CI, confidence interval; CRP, C-reactive protein; ETD, estimated treatment difference; NA, not assessed; T2D, type 2 diabetes. aParticipant numbers represent the number of participants in the full analysis set (the number contributing to the analysis of estimated changes). bBaseline values are observed data for the total population among participants with a baseline CRP assessment.