Figure 5. Effect of signal peptide on folding of the twins.
-
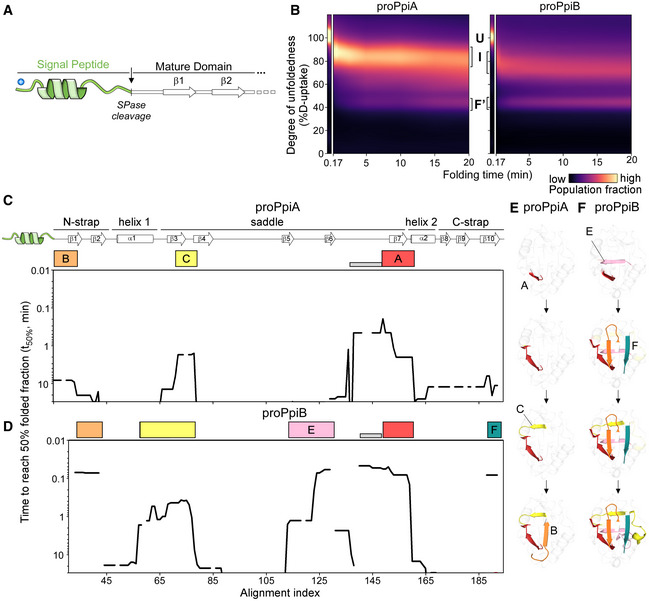
ALinear map of the signal peptide/early mature domain region of proPpiA.
- B
-
C, DFolding kinetics of proPpiA and proPpiB, at 25°C, monitored by local HDX‐MS (Dataset EV4; n = 3 biological repeats), were analysed by PyHDX to determine the folded fractions per residue (Dataset EV5). The time needed to reach 50% of folded fraction (t 50% values; only for the mature domains shown here) was plotted as in Fig 3; see extended dataset colour map in Appendix Fig S3.
-
E, FFoldons, coloured (as in C, D) on the PpiA (1V9T; E) and PpiB (1LOP; F) 3D structures. The indicated time points are as follows: for proPpiA (t 50% of 0.9‐2.0‐2.3‐20.8 min) and for proPpiB (t 50% of 0.06‐0.08‐0.44‐1.2 min; Dataset EV5).
Source data are available online for this figure.
