Abstract
Study Objectives:
Patients with obstructive sleep apnea (OSA) show brain injury in sites responsible for autonomic, cognitive, and respiratory functions. Brain changes in OSA may vary with disease severity as assessed by the apnea-hypopnea index (AHI), which does not provide information about the apnea depth and length in contrast to oxygen desaturation. Although significant associations with brain injury and AHI are known in OSA, it is unclear whether AHI or the extent of oxygen desaturations better correlate with brain damage. We evaluated associations between brain changes, AHI, and oxygen desaturation using diffusion tensor imaging–based measures.
Methods:
We acquired diffusion tensor imaging data from 19 patients with OSA using a 3.0-Tesla MRI scanner and calculated, normalized, and smoothed mean, axial, and radial diffusivity maps that were used for correlations between brain changes, oxygen desaturation, and AHI values.
Results:
Positive correlations with extent of injury (mean, axial, and radial diffusivity values) and AHI appeared in the frontal areas, cingulate and insula, amygdala, hippocampus, and basal pons, and negative associations emerged in the putamen, internal-capsule, globus-pallidus, and cerebellar cortices. Regional diffusivity values and oxygen desaturation showed positive correlations in the cingulate, frontal, putamen, and cerebellar sites, and negative relationships in several areas, including the occipital cortex.
Conclusions:
Patients with OSA show negative and positive correlations, indicated by increased and decreased diffusivity values, resulting from chronic and acute changes in those areas. The extent of injury in OSA partially depends on the extent of AHI and oxygen desaturation, with the effects representing continued development from acute to chronic processes.
Citation:
Sahib A, Roy B, Kang D, Aysola RS, Wen E, Kumar R. Relationships between brain tissue damage, oxygen desaturation, and disease severity in obstructive sleep apnea evaluated by diffusion tensor imaging. J Clin Sleep Med. 2022;18(12):2713–2721.
Keywords: issue injury, mood, autonomic, cognition, disease severity, hippocampus
BRIEF SUMMARY
Current Knowledge/Study Rationale: Obstructive sleep apnea patients show brain injury in various sites and these tissue changes may vary with disease severity, as assessed by the apnea-hypopnea index (AHI) and oxygen desaturation. However, it is unclear whether AHI or the extent of oxygen desaturations better correlate with brain changes.
Study Impact: We evaluated associations between brain tissue integrity, AHI, and oxygen desaturation using diffusion tensor imaging–based measures and showed both negative and positive correlations, indicated by increased and decreased in diffusivity values, resulting from chronic and acute changes, suggesting continued development from acute to chronic processes. In addition, AHI showed more widespread associations than did oxygen desaturation, indicating AHI to be more reliable than oxygen desaturation.
INTRODUCTION
Obstructive sleep apnea (OSA) is a common disorder resulting from loss of upper airway muscle coordination with diaphragmatic efforts during sleep, leading to multiple intermittent hypoxic episodes that result in brain changes. Multiple magnetic resonance imaging (MRI) procedures have shown brain injury resulting from the intermittent hypoxia episodes in several autonomic, mood, and cognitive control areas; these injuries may vary with disease severity, as assessed by apnea-hypopnea index (AHI) and oxygen desaturation indices. These MRI studies have shown significant associations between brain damage and AHI, but it is unclear whether AHI or oxygen desaturation indices better correlate with brain damage in newly diagnosed, treatment-naïve patients with OSA.
The primary index used in determination of severity of OSA is AHI, which describes the number of apneas and hypopneas per sleep hour.1 Both apneas and hypopneas contribute equally to AHI calculations and the determination of OSA severity, although their characteristics are very different.2 Apnea or hypopnea event durations vary between patients with OSA with a similar AHI,3 which may introduce variable hypoxic/ischemic loads that contribute to differing extents of brain changes.
In addition to AHI, the respiratory abnormality in OSA is also characterized by the degree of oxygen saturation (SaO2), although its reliability for OSA severity remains unclear.4 The extent of oxygen desaturation depends on the baseline oxygen saturation, lung volume, and apnea duration in the condition.5 Oxygen saturation can reach significantly low values in OSA,4,6 which can contribute to similar hypoxia-dose responses in brain tissue as repeated intermittent hypoxia events lead to ischemia-reperfusion injury and contribute to increased production of reactive oxygen species and oxidative stress.7 Therefore, there is a need to understand the effect of oxygen desaturation on brain damage in the OSA population.
Multiple MRI procedures, including diffusion tensor imaging (DTI), which measures motion of water molecules within tissue and is influenced by various factors, including tissue barriers and extracellular/extra-axonal fluid and space, have shown brain changes in patients with OSA. Based on DTI procedures, several indices, including mean diffusivity (MD), which measures the average motion of water molecules within tissue, axial and radial diffusivity (AD and RD), which measure diffusion parallel and perpendicular to axons, can be calculated and have provided useful assessments of brain injury in OSA.8,9 At acute stages of pathology after hypoxemia, cytotoxic edematous actions decrease extracellular water and lead to cell and axonal swelling, reducing extracellular space, resulting in restricted water diffusion and, thus, reduced MD, AD, and RD values. However, demyelination or axonal loss in chronic stages of hypoxemia reduce tissue barriers, increase extracellular volume, and escalate vasogenic edema, factors that contribute to increased MD, AD, and RD values.10,11 However, the relationships between whole-brain diffusivity measures with severity of disease in the context of AHI and oxygen desaturation (SaO2) have not been explored.
Our aim was to evaluate correlations between brain changes, AHI, and SaO2 values in recently-diagnosed, treatment-naïve patients with OSA by using DTI-based measures. We hypothesized that both AHI and SaO2 would show sites with variable correlations with brain changes in patients with OSA.
METHODS
Patients
Nineteen newly-diagnosed (age 5.4 ± 9.2; body-mass-index [BMI], 3.6 ± 6.7 kg/m2; males, 14), treatment-naïve patients with OSA were recruited from the Sleep Disorders Laboratory at the University of California Los Angeles. All patients had undergone overnight polysomnography and had a moderate-to-severe diagnosis (AHI ≥ 15 events/h), with no history of neurological illness or psychiatric disorders other than OSA condition. Patients with metallic implants incompatible with the MRI scanner environment, a history of stroke or traumatic brain injury, heart failure, lung parenchymal disease, atrial septal defect, ventricular septal defect, or psychiatric diseases were excluded from the study. All patients provided written informed consent before undergoing the study procedures, which were conducted in accordance with approval from the Institutional Review Board at UCLA.
Overnight polysomnography
Overnight polysomnography data were analyzed following American Academy of Sleep Medicine 1A criteria by a registered sleep physician at the UCLA Sleep Disorders Clinic. Disease severity indices included AHI and oxygen saturation change values. AHI was defined as the ratio of the total number of hypopneas and apneas over the total number of sleep in hours. Patients with OSA with an AHI ≥ 15 and < 30 were classified as moderate, while those with an AHI ≥ 30 were classified as severe. Oxygen desaturation values were reported based on overnight polysomnography studies, and the lowest point of oxygen saturation values were represented as SaO2 nadir. The difference between the baseline oxygen saturation and SaO2 nadir (SaO2 change) was calculated.
Assessment of sleep and neuropsychologic functions
To investigate sleep quality and daytime sleepiness, we employed 2 self-administered questionnaires,12 including the Pittsburgh Sleep Quality Index and Epworth Sleepiness Scale, respectively. For assessment of anxiety13 and depressive14 symptoms in patients with OSA, we used the Beck Anxiety Inventory (BAI) and Beck Depression Inventory (BDI) questionnaires. The BAI and BDI tools are self-administered questionnaires on symptom severity. Each questionnaire consists of 21 questions with answers ranging in value from 0 to 3 and total score ranging from 0 to 63.
Cognition assessment
We performed the Montreal Cognitive Assessment test for cognition examination. This test includes fast screening of various cognitive domains, including attention and concentration, language, memory, executive functions, visuo-constructional skills, conceptual thinking, calculations, and orientation. In addition, the Trial Making Tests A and B were also conducted, which are used for evaluating of dementia, including executive function, visual search, mental flexibility, scanning, and speed of processing.
Magnetic resonance imaging
Brain images from all OSA patients were acquired using a 3.0-Tesla MRI scanner (Magnetom Trio, A Tim System; Siemens, Erlangen, Germany). Foam pads were used on both sides of the head to minimize head motion during data acquisition. High-resolution T1-weighted images were collected using a magnetization-prepared rapid acquisition gradient echo (MPRAGE) sequence (repetition time = 2200 ms; echo time = 2.4 ms; inversion time = 900 ms; flip angle [FA] = 9°; matrix size = 320 × 320; field of view [FOV] = 230 × 230 mm; slice thickness = .9 mm; number of slices = 192). Proton-density and T2-weighted images (repetition time = 10,000 ms; echo time 1,2 = 17, 134 ms; FA = 130°) were also acquired simultaneously using a dual-echo turbo spin-echo sequence in the axial plane, with a 256 × 256 matrix size, 230 × 230 mm FOV, 4-mm slice thickness, and no interslice gap. DTI data were collected using a single-shot echo planer imaging with twice-refocused spin-echo pulse sequence (repetition time = 12,000 ms; echo time = 87 ms; FA = 90°; band width = 1,347 Hz/pixel; matrix size = 128 × 128; FOV = 230 × 230 mm; slice thickness = 1.7 mm, diffusion values = 0 and 700 s/mm2, diffusion directions = 64, separate series = 2).
Data processing
The statistical parametric mapping package SPM12 (https://www.fil.ion.ucl.ac.uk/spm/), Diffusion Toolkit,15 and MATLAB-based (https://www.mathworks.com/) custom software were used for data processing and analyses. Diffusion- and non–diffusion weighted images of all patients with OSA were assessed for any head-motion related or other imaging artifacts before DTI indices calculations.
DTI indices calculations and processing
Diffusion tensor metrics were calculated with Diffusion Toolkit software using diffusion-weighted (b = 700 s/mm2) and non–diffusion weighted images (b = 0 s/mm2). Using the diffusion tensor matrices, principal eigen values (λ1, λ2, and λ3) were calculated,16 and these eigen values were used to derive MD [λ = (λ1 + λ2 + λ3)/3], AD (λ‖‖ = λ1), and RD [λ⊥ = (λ2 + λ3)/2] values at each voxel,16,17 with voxel intensities on the MD, AD, and RD maps showing the corresponding diffusion values. We used a fixed threshold value to mask out nonbrain regions and background noise.
The MD, AD, and RD maps derived from each DTI series of patients with OSA were realigned to remove any potential variation from head-motion and averaged to increase signal. Similarly, non–diffusion weighted images were also realigned and averaged. The averaged MD, AD, and RD maps of all the patients with OSA were normalized to standard brain Montreal Neurological Institute (MNI) template space, using non–diffusion weighted images. Non–diffusion weighted (b0) images were normalized first to MNI space using a unified segmentation approach,18 and resulting normalization parameters were applied to corresponding MD, AD, and RD maps. The normalized MD, AD, and RD maps were smoothed with a Gaussian filter (10 mm).
Global brain mask
The averaged T1-weighted images of each patient were partitioned into gray matter, white matter, and cerebrospinal fluid tissue types. The gray and white matter probability maps of all patients were normalized into MNI space and averaged to create a global gray matter and a white matter probability map. A probability threshold of 0.3 was applied to the averaged gray and white matter probability maps, which were then combined to form a whole-brain brain mask.
Background images
High-resolution T1-weighted images of a control patient were normalized to MNI space, using the modified unified segmentation approach. The normalized images were used as the background images to overlay findings for structural identification.
Region-of-interest analyses
Region-of-interest analyses were performed to calculate magnitude of correlations between regional brain MD, AD, and RD values and AHI and SaO2 change in patients with OSA. Using a neuromorphometric atlas (http://www.neuromorphometrics.com), brain site–specific masks were created based on findings from partial correlation analysis. In addition, manual brain masks were created for sites that were not available in the neuromorphometric atlas using MRIcron (NeuroImaging Tools and Resources Collaboratory; https://www.nitrc.org/projects/mricron). These anatomic-specific brain masks were used to compute correlation values between smoothed MD, AD, RD maps and AHI and SaO2 change.
Statistical analyses
We used the SPM12 and the IBM Statistical Packages for the Social Sciences (SPSS v24; Armonk, NY) software for statistical analyses. We examined significant correlations between regional brain tissue integrity and disease severity in patients with OSA voxel-by-voxel using the smoothed MD maps and AHI and SaO2 change values with partial correlations (SPM12; covariates, age and sex; uncorrected threshold, P < .005). We used the global brain mask to restrict correlation calculations within gray and white matter regions. We overlaid clusters with significant correlations onto background images for structural identification.
Correlation coefficients were calculated using regional MD, AD, and RD values, determined by region-of-interest analyses, based on correlations between whole-brain MD maps and AHI and SaO2 change (IBM SPSS software; partial correlations; covariates, age and sex). In addition, whole-brain effect size (r-map) maps were also generated using the partial correlation procedures implemented in REST toolbox,19 with age and sex as covariates. A P-value of less than .05 was used to establish statistical significance.
RESULTS
Brain sites with correlations between MD and AHI values
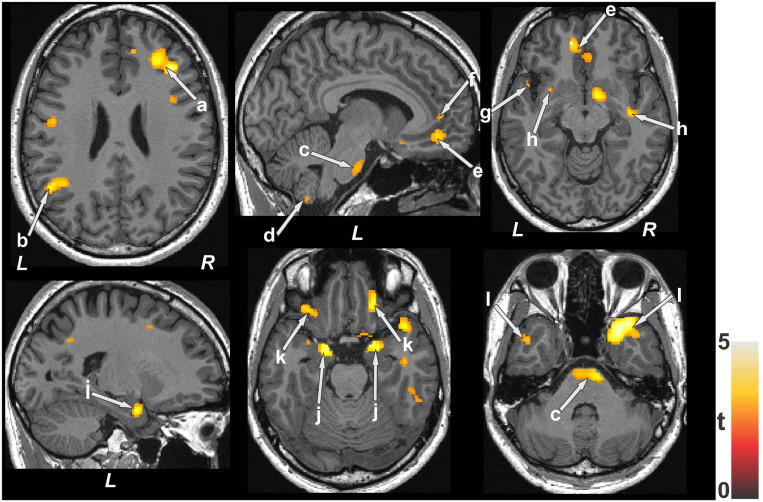
The average AHI score recorded across patients with OSA was 4.3 ± 22.9 events/h. Positive correlations emerged in patients with OSA between AHI and MD values in various brain sites, including the frontal (Figure 1a) and parietal cortices (Figure 1b), basal pons (Figure 1c), cerebellar cortices (Figure 1d), ventral medial prefrontal cortices (Figure 1e), anterior cingulate (Figure 1f) and insula (Figure 1g), internal capsule (Figure 1h), hippocampus (Figure 1i), caudate, amygdala (Figure 1j), basal forebrain, prefrontal cortices (Figure 1k), and ventral temporal lobes (Figure 1l). Negative associations appeared in different brain regions, including the parietal cortex (Figure 2a), putamen (Figure 2b), globus pallidus (Figure 2c), internal capsule (Figure 2d), and cerebellar cortices (Figure 2e).
Figure 1. Brain sites with significant positive correlations between MD values and AHI scores in patients with OSA.
These sites include the frontal cortices (a), parietal cortices (b), basal pons (c), cerebellar cortices (d), bilateral ventral medial prefrontal cortices (e), anterior cingulate (f), insula (g), internal capsule cortices (h), hippocampus (i), amygdala (j), prefrontal cortices (k), and ventral temporal lobes (l). Color bar represents t statistic values (L = left; R = right). AHI = apnea-hypopnea index, MD = mean diffusivity, OSA = obstructive sleep apnea.
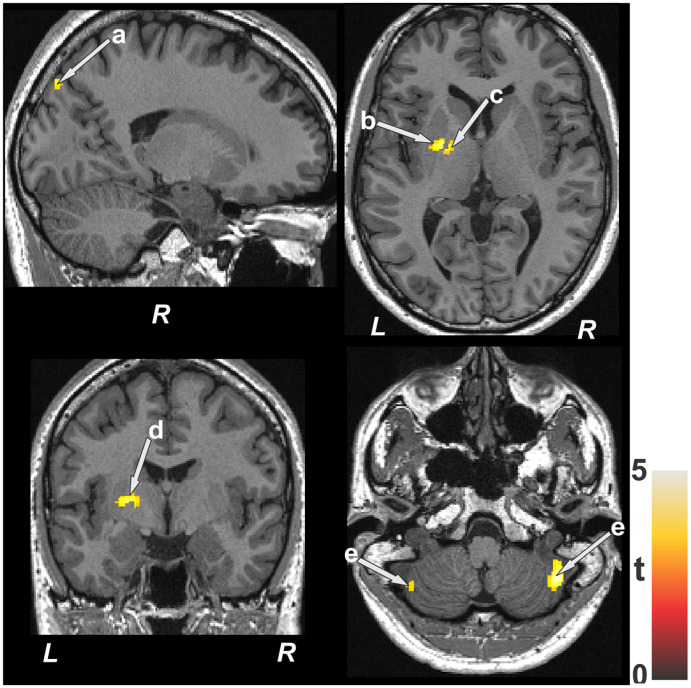
Figure 2. Brain sites with significant negative correlations between MD values and AHI scores in patients with OSA.
These areas included the parietal cortex (a), putamen (b), globus pallidus (c), internal capsule (d), and cerebellar cortices (e). Color bar represents t statistic values (L = left; R = right). AHI = apnea-hypopnea index, MD = mean diffusivity, OSA = obstructive sleep apnea.
Brain areas with correlations between MD and SaO2 values
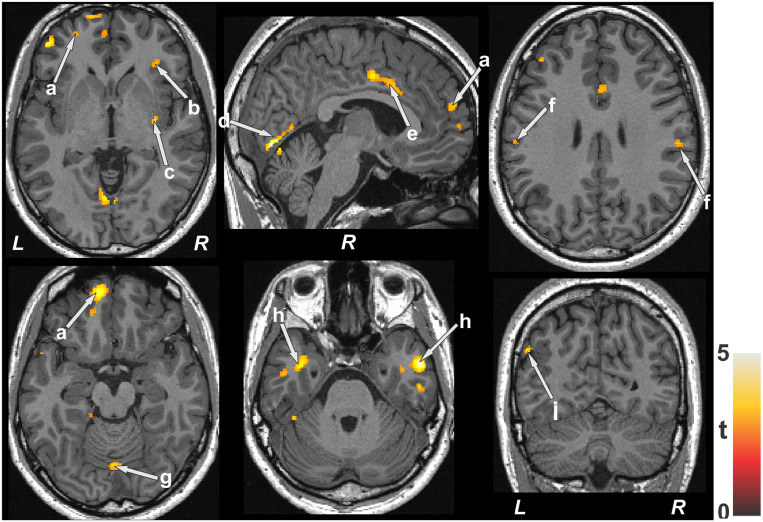
The average oxygen saturation change value of patients with OSA was 15.8 ± 7.9%. Positive correlations between MD and O2 saturation change values emerged in the bilateral frontal and medial frontal areas (Figure 3a), right anterior insula (Figure 3b), putamen and external capsule (Figure 3c), occipital cortex (Figure 3d), mid-cingulate (Figure 3e), parietal cortices (Figure 3f), cerebellar vermis (Figure 3g), and ventral temporal cortices (Figure 3h), and negative relationships appeared in the occipital cortex (Figure 3i).
Figure 3. Brain sites with significant positive correlations between MD values and SaO2 delta scores in patients with OSA.
These sites included the frontal cortices (a), insular cortices (b), putamen (c), occipital cortex (d), mid-cingulate (e), parietal cortices (f), cerebellar vermis (g), temporal cortices (h), and occipital cortex (i). Color bar represents t statistic values (L = left; R = right). MD = mean diffusivity, OSA = obstructive sleep apnea.
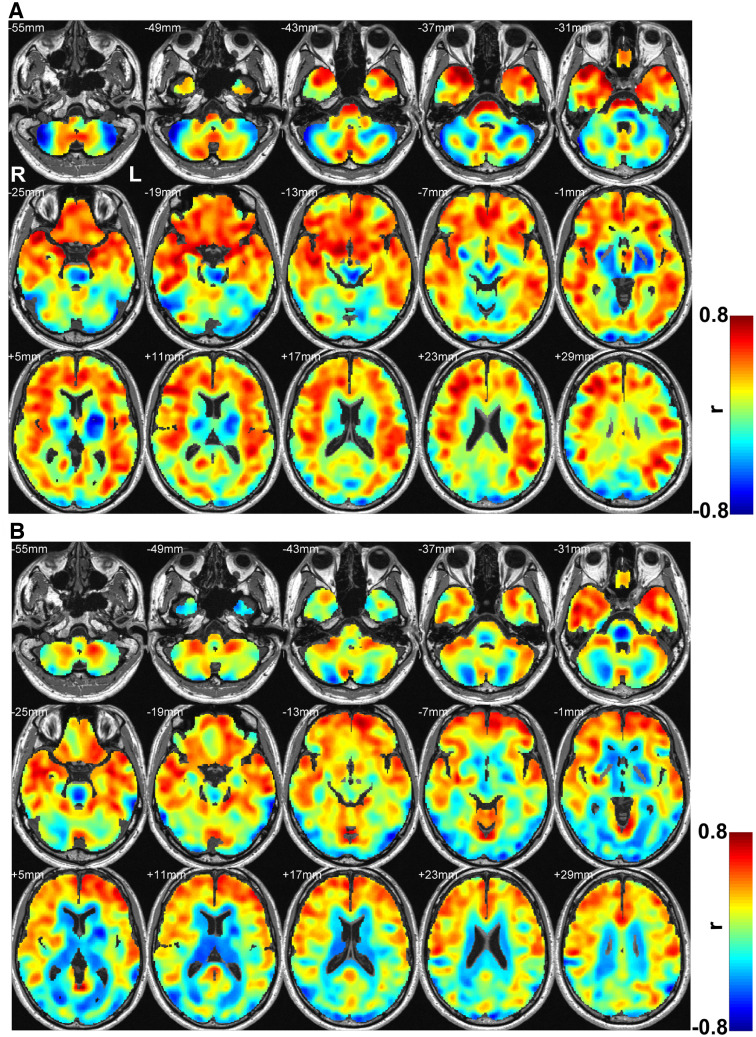
Whole-brain correlation effect sizes between MD and AHI and SaO2 change values
AHI showed higher and more widespread effect sizes (Figure 4A) over SaO2 values (Figure 4B) with regional brain MD values in patients with OSA. The frontal, medial, and prefrontal areas, as well as insula, cingulate, hippocampus, caudate, amygdala, parietal, and cerebellar cortices showed more positive associations, while negative correlations were observed in the thalamus, putamen, globus pallidus, and lateral occipital regions with AHI over SaO2 values.
Figure 4. Effect size maps between MD and AHI (A) and MD and SaO2 delta (B).
Color bar represents r-values (L = left; R = right). AHI = apnea-hypopnea index, MD = mean diffusivity.
Regional brain MD, AD, and RD, as well as clinical variables correlations with AHI and SaO2 values
Regional brain correlation coefficients between MD, AD, and RD and AHI and SaO2 delta values are listed in Table 1. Compared to AD, RD values showed higher correlations with AHI and SaO2 values.
Table 1.
Regional brain correlation (r) values between DTI measures, and AHI scores and SaO2 changes in patients with OSA.
| Brain Regions | MD vs AHI r (P-value) | AD vs AHI r (P-value) | RD vs AHI r (P-value) | MD vs SaO2 r (P-value) | AD vs SaO2 r (P-value) | RD vs SaO2 r (P-value) |
|---|---|---|---|---|---|---|
| L Insula | .65 (.005) | .66 (.004) | .65 (.005) | – | – | – |
| R Amygdala | .70 (.002) | .70 (.002) | .68 (.003) | – | – | – |
| L Amygdala | .70 (.002) | .71 (.001) | .69 (.002) | – | – | – |
| R Basal forebrain | .72 (.001) | .71 (.001) | .72 (.001) | – | – | – |
| L Basal forebrain | .68 (.003) | .69 (.002) | .68 (.003) | – | – | – |
| R Mid-cingulate | – | – | – | .68 (.003) | .67 (.003) | .68 (.003) |
| L Mid-cingulate | – | – | – | .67 (.003) | .64 (.006) | .68 (.003) |
| R Medial orbital gyrus | .74 (.001) | .73 (.001) | .74 (.001) | – | – | – |
| L Medial orbital gyrus | – | – | – | .68 (.003) | .67 (.003) | .69 (.002) |
| R Temporal pole | .79 (.0001) | .78 (.0001) | .79 (.0001) | .69 (.002) | .65 (.004) | .70 (.002) |
| L Temporal pole | .78 (.0001) | .76 (.0003) | .78 (.0001) | .70 (.002) | .68 (.003) | .70 (.002) |
| L Medial frontal cortex | .66 (.004) | .67 (.004) | .66 (.004) | .68 (.003) | .66 (.004) | .68 (.002) |
| R Middle temporal gyrus | – | – | – | .76 (.0004) | .71 (.001) | .79 (.0001) |
| L Middle temporal gyrus | .66 (.004) | .71 (.002) | .64 (.006) | .72 (.001) | .70 (.002) | .74 (.001) |
| Cerebellar cortices | .63 (.006) | .63 (.007) | .64 (.006) | – | – | – |
| Cerebellar vermis | – | – | – | .68 (.003) | .65 (.004) | .68 (.003) |
| Basal pons | .68 (.003) | .67 (.003) | .68 (.002) | – | – | – |
| R Putamen | – | – | – | .66 (.004) | – | .63 (.007) |
| L Putamen | –.69 (.002) | –.62 (.008) | –.64 (.006) | – | – | – |
| Parietal cortex | –.67 (.003) | –.59 (.013) | –.69 (.002) | – | – | – |
| Cerebellar cortices II | –.75 (.0001) | –.75 (.001) | –.73 (.001) | – | – | – |
| Occipital cortex | – | – | – | –.68 (.002) | –.66 (.004) | –.66 (.004) |
AD = radial diffusivity, L = left, MD = mean diffusivity, OSA = obstructive sleep apnea, R = right, RD = radial diffusivity.
AHI showed significant negative correlation with Pittsburgh Sleep Quality Index and Trail B, and SaO2 delta showed significant positive correlation with BMI (Table 2). Other physiologic, neuropsychologic, sleep, and cognitive variables did not show any significant relationships with AHI or SaO2 delta values.
Table 2.
Correlation of OSA characteristics, symptoms with AHI and SaO2 delta.
| Physiological Variable (mean ± SD) | AHI r (P-value) | SaO2 Delta r (P-value) |
|---|---|---|
| PSQI (6.6 ± 2.9) | –.533 (.027) | –.092 (.725) |
| ESS (8.0 ± 4.2) | .199 (.443) | .390 (.122) |
| BAI (5.3 ± 5.6) | –.087 (.740) | –.218 (.401) |
| BDI-II (5.6 ± 4.7) | –.275 (.285) | –.386 (.126) |
| MoCA (25.5 ± 4.0) | .264 (.306) | –.051 (.846) |
| BMI (30..63 ± 6.69) | .377 (.136) | .580 (.015) |
| Trail A (26.49 ± 7.98) | –.462 (.062) | –.052 (.842) |
| Trail B (69.18 ± 33.94) | –.531 (.028) | .261 (.312) |
AHI = apnea–hypopnea–index, BAI = Beck Anxiety Inventory, BDI = Beck Depression Inventory, BMI = body mass index, ESS = Epworth Sleepiness Scale, MoCA= Montreal Cognitive Assessment, OSA = obstructive sleep apnea, PSQI = Pittsburgh Sleep Quality Index, SaO2 = oxygen saturation, SD = standard deviation.
DISCUSSION
Overview
Patients with OSA show positive relationships between AHI and DTI metrics in various brain regions, including the insula, basal forebrain, amygdala, frontal, parietal and temporal cortices. Since newly-diagnosed, treatment-naïve patients with OSA show reduced DTI metrics, including MD, AD, and RD values, positive relationships between AHI and DTI metrics indicate acute brain injury in those sites. In addition, few areas such as parietal, putamen, globus pallidus, and cerebellar cortices showed negative associations between AHI and DTI measures, suggesting chronic tissue damage in those regions. Similarly, predominantly positive relationships emerged between DTI metrics and SaO2 change in brain regions including the insula, putamen, cingulate, basal forebrain, vermis, frontal, and temporal cortices, indicating acute tissue damage in those areas. However, AHI showed more widespread correlations and consequently higher effect sizes compared to SaO2 delta values with DTI indices. In addition, RD showed better correlation than AD, suggesting predominant myelin changes over axons in patients with OSA.
OSA and brain injury
Diffusion tensor imaging–based measures are sensitive to examine microstructural changes in the brain and can assist in tissue characterization,17,20 as used successfully in various pathological fiber determination in various disorders.21–23 MD procedures can distinguish acute from chronic tissue changes, with decreased MD values in acute stages, and increased MD values in chronic stages.10,11 Patients with OSA experience repetitive episodes of hypoxia/reoxygenation during transient cessation of breathing that lead to oxidative stress and inflammation.24,25 As a result, both axons and myelin are damaged in recently diagnosed, untreated patients with OSA, with more myelin damage over axons, as indicated by significantly reduced global and regional DTI measures.8,9,26 Injured brain sites include the frontal, parietal, temporal, insular, amygdala, thalamus, and cerebellar regions, areas that are responsible for mood, autonomic, and cognitive functions. In this study, several white matter and subcortical sites demonstrated positive and negative correlations with AHI and SaO2 change, including the frontal, parietal, amygdala, insular, and cerebellar regions, indicating acute and chronic tissue injury with disease severity. These findings indicate that DTI-based measures are effective for evaluating associations between brain tissue integrity and disease severity in patients with OSA.
AHI vs oxygen desaturation indices
AHI has been defined as the measure of OSA severity by the American Academy of Sleep Medicine for a long time.27 However, recent studies have shown that AHI is not the most appropriate measure of clinical impact for OSA.28,29 In addition, it is unclear whether AHI reflects better impact on brain changes in the condition. One possible reason for not reflecting OSA clinical symptoms may be that AHI is based on total number of apneas and hypopneas, without severity of apneic events. Nocturnal intermittent oxygen desaturation can increase sympathetic vasoconstriction, leading to increase blood pressure and changes in structure and function of brain blood vessels,30 contributing to alterations in regional cerebral blood flow. Therefore, some studies have suggested that oxygen desaturation indices are a better predictor than AHI values of clinical symptoms, including daytime sleepiness31 and cardiovascular sequelae.32,33 Although previous studies34,35 have shown the relationships between AHI and brain MRI indices, it is unclear whether AHI or oxygen desaturation better correlates in newly diagnosed OSA with brain tissue integrity assessed by DTI indices.
Positive and negative correlations vs disease severity indices
Both positive and negative correlations emerged between regional MD values and AHI and SaO2 values in newly diagnosed patients with OSA. Our previous studies showed acute tissue changes with reduced MD values and predominantly myelin alteration in newly diagnosed (lowered AD and RD values), treatment-naïve patients with OSA in several brain regions that regulate autonomic, respiratory, mood, and cognitive functions.8,9 The findings of positive correlations suggest that patients with OSA with lower AHI or SaO2 values at the beginning of condition have predominately acute tissue changes. However, negative associations suggest progression of injury in those areas to chronic stage with higher AHI and SaO2 in long-term in the condition.
Positive correlations appeared between AHI, SaO2, and MD values in several white and gray matter regions in the frontal and temporal cortices, sites that contribute significantly to cognitive deficits, sleep loss, and fragmented sleep.36,37 Similarly, positive correlations appeared in insular and cingulate cortices between AHI, SaO2, and DTI indices. Injury to these regions can establish the basis for affective disorders, including depression and anxiety, and leads to deficits in various autonomic and baroreceptive functions38–41 that have been reported in OSA.9,40 Cognitive and mood dysfunctions commonly reported in patients with OSA may result from previously reported acute tissue injury in brain regions, including the amygdala and basal forebrain,8 areas that also showed a significant positive relationship with disease severity symptoms. In addition, the midline pons and cerebellar cortices that are essential for regulation of blood pressure, respiration, and integration of baroreceptor and chemoreceptor afferents,42,43 showed positive associations with AHI and SaO2 change values.
In addition to positive associations, negative relationships emerged between several brain sites, including the parietal cortex, putamen, globus pallidus, external capsule, cerebellar cortices, and occipital cortex and AHI and SaO2 change values. These relations indicate that brain sites with acute tissue injury have progressed to a chronic stage severely affecting various autonomic and motor functions, which are deficient in the condition.
AHI showed more widespread correlations over SaO2
Compared to SaO2 change, AHI showed a higher effect size in several brain regions, including the frontal, insula, cingulate, hippocampus, amygdala, and parietal cortices. These findings indicate AHI to be more concordant than SaO2 with brain changes in OSA.
Limitations
Several limitations need to be acknowledged in this study including the small number of patients with OSA and potential contributions of BMI on brain changes. The present results are limited to newly diagnosed, untreated, middle-aged patients with OSA, without any major comorbid illness, so different patterns may appear in other OSA populations. In addition, the findings need to be repeated on a larger population with varying disease severity, controlling for BMI as a confounding factor, and approaching with a specific hypothesis-driven region-of-interest analysis methodology.
CONCLUSIONS
Significant positive and negative correlations emerged between DTI-based indices and AHI scores in newly diagnosed, treatment-naïve patients with OSA, indicating disease-dependent altered tissue changes in the condition. Positive associations that may lead to decrease in diffusivity values appeared in autonomic, cognitive, and neuropsychologic control areas, including the insula, amygdala, basal forebrain, midline pons, frontal, temporal, parietal and cerebellar cortices, indicating acute tissue damage in these areas. Negative associations that can lead to an increase in diffusivity values emerged between AHI and DTI indices in the parietal cortex, putamen, globus pallidus, external capsule, cerebellar cortices, and occipital cortex, indicating chronic tissue injury. Oxygen desaturation values showed positive associations with comparable brain areas as AHI.
ACKNOWLEDGMENTS
The authors thank Mr. Luke Ehlert, Ms. Milena Lai, and Mrs. Karen Harada for assistance with data collection.
ABBREVIATIONS
- AD
axial diffusivity
- AHI
apnea-hypopnea index
- BMI
body mass index
- DTI
diffusion tensor imaging
- MD
mean diffusivity
- MNI
Montreal Neurological Institute
- MRI
magnetic resonance imaging
- OSA
obstructive sleep apnea
- RD
radial diffusivity
- SaO2
oxygen saturation
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. Institution where work was performed: University of California Los Angeles, Los Angeles. This research work was supported by National Institutes of Health R01 HL-113251 and R01 NR-015038. Ashish Sahib, Bhaswati Roy, and Daniel Kang were supported by R01 NR-015038. Rajesh Kumar was supported by (R01 HL-113251 and R01 NR-015038). The authors report no conflicts of interest.
REFERENCES
- 1. Silber MH , Ancoli-Israel S , Bonnet MH , et al . The visual scoring of sleep in adults . J Clin Sleep Med. 2007. ; 3 ( 2 ): 121 – 131 . [PubMed] [Google Scholar]
- 2. Punjabi NM . COUNTERPOINT: is the apnea-hypopnea index the best way to quantify the severity of sleep-disordered breathing? No . Chest. 2016. ; 149 ( 1 ): 16 – 19 . [DOI] [PubMed] [Google Scholar]
- 3. Muraja-Murro A , Nurkkala J , Tiihonen P , et al . Total duration of apnea and hypopnea events and average desaturation show significant variation in patients with a similar apnea-hypopnea index . J Med Eng Technol. 2012. ; 36 ( 8 ): 393 – 398 . [DOI] [PubMed] [Google Scholar]
- 4. Netzer N , Eliasson AH , Netzer C , Kristo DA . Overnight pulse oximetry for sleep-disordered breathing in adults: a review . Chest. 2001. ; 120 ( 2 ): 625 – 633 . [DOI] [PubMed] [Google Scholar]
- 5. Sériès F , Cormier Y , La Forge J . Influence of apnea type and sleep stage on nocturnal postapneic desaturation . Am Rev Respir Dis. 1990. ; 141 ( 6 ): 1522 – 1526 . [DOI] [PubMed] [Google Scholar]
- 6. Ling IT , James AL , Hillman DR . Interrelationships between body mass, oxygen desaturation, and apnea-hypopnea indices in a sleep clinic population . Sleep. 2012. ; 35 ( 1 ): 89 – 96 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dewan NA , Nieto FJ , Somers VK . Intermittent hypoxemia and OSA: implications for comorbidities . Chest. 2015. ; 147 ( 1 ): 266 – 274 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kumar R , Pham TT , Macey PM , Woo MA , Yan-Go FL , Harper RM . Abnormal myelin and axonal integrity in recently diagnosed patients with obstructive sleep apnea . Sleep. 2014. ; 37 ( 4 ): 723 – 732 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kumar R , Chavez AS , Macey PM , Woo MA , Yan-Go FL , Harper RM . Altered global and regional brain mean diffusivity in patients with obstructive sleep apnea . J Neurosci Res. 2012. ; 90 ( 10 ): 2043 – 2052 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ahlhelm F , Schneider G , Backens M , Reith W , Hagen T . Time course of the apparent diffusion coefficient after cerebral infarction . Eur Radiol. 2002. ; 12 ( 9 ): 2322 – 2329 . [DOI] [PubMed] [Google Scholar]
- 11. Matsumoto K , Lo EH , Pierce AR , Wei H , Garrido L , Kowall NW . Role of vasogenic edema and tissue cavitation in ischemic evolution on diffusion-weighted imaging: comparison with multiparameter MR and immunohistochemistry . AJNR Am J Neuroradiol. 1995. ; 16 ( 5 ): 1107 – 1115 . [PMC free article] [PubMed] [Google Scholar]
- 12. Knutson KL , Rathouz PJ , Yan LL , Liu K , Lauderdale DS . Stability of the Pittsburgh Sleep Quality Index and the Epworth Sleepiness Questionnaires over 1 year in early middle-aged adults: the CARDIA study . Sleep. 2006. ; 29 ( 11 ): 1503 – 1506 . [DOI] [PubMed] [Google Scholar]
- 13. Beck AT , Epstein N , Brown G , Steer RA . An inventory for measuring clinical anxiety: psychometric properties . J Consult Clin Psychol. 1988. ; 56 ( 6 ): 893 – 897 . [DOI] [PubMed] [Google Scholar]
- 14. Beck AT , Steer RA , Ball R , Ranieri W . Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients . J Pers Assess. 1996. ; 67 ( 3 ): 588 – 597 . [DOI] [PubMed] [Google Scholar]
- 15. Zhang H , Yushkevich PA , Alexander DC , Gee JC . Deformable registration of diffusion tensor MR images with explicit orientation optimization . Med Image Anal. 2006. ; 10 ( 5 ): 764 – 785 . [DOI] [PubMed] [Google Scholar]
- 16. Basser PJ , Pierpaoli C . Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI . J Magn Reson B. 1996. ; 111 ( 3 ): 209 – 219 . [DOI] [PubMed] [Google Scholar]
- 17. Le Bihan D , Mangin JF , Poupon C , et al . Diffusion tensor imaging: concepts and applications . J Magn Reson Imaging. 2001. ; 13 ( 4 ): 534 – 546 . [DOI] [PubMed] [Google Scholar]
- 18. Ashburner J , Friston KJ . Unified segmentation . Neuroimage. 2005. ; 26 ( 3 ): 839 – 851 . [DOI] [PubMed] [Google Scholar]
- 19. Song XW , Dong ZY , Long XY , et al . REST: a toolkit for resting-state functional magnetic resonance imaging data processing . PLoS One. 2011. ; 6 ( 9 ): e25031 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kumar R , Macey PM , Woo MA , Alger JR , Harper RM . Elevated mean diffusivity in widespread brain regions in congenital central hypoventilation syndrome . J Magn Reson Imaging. 2006. ; 24 ( 6 ): 1252 – 1258 . [DOI] [PubMed] [Google Scholar]
- 21. Betz J , Zhuo J , Roy A , Shanmuganathan K , Gullapalli RP . Prognostic value of diffusion tensor imaging parameters in severe traumatic brain injury . J Neurotrauma. 2012. ; 29 ( 7 ): 1292 – 1305 . [DOI] [PubMed] [Google Scholar]
- 22. Blaschek A , Keeser D , Müller S , et al . Early white matter changes in childhood multiple sclerosis: a diffusion tensor imaging study . AJNR Am J Neuroradiol. 2013. ; 34 ( 10 ): 2015 – 2020 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ryan NS , Keihaninejad S , Shakespeare TJ , et al . Magnetic resonance imaging evidence for presymptomatic change in thalamus and caudate in familial Alzheimer’s disease . Brain. 2013. ; 136 ( Pt 5 ): 1399 – 1414 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jelic S , Le Jemtel TH . Inflammation, oxidative stress, and the vascular endothelium in obstructive sleep apnea. 2008. ;18(7):253–260. [DOI] [PubMed]
- 25. Sforza E , de Saint Hilaire Z , Pelissolo A , Rochat T , Ibanez V . Personality, anxiety and mood traits in patients with sleep-related breathing disorders: effect of reduced daytime alertness . Sleep Med. 2002. ; 3 ( 2 ): 139 – 145 . [DOI] [PubMed] [Google Scholar]
- 26. Macey PM , Kumar R , Woo MA , Valladares EM , Yan-Go FL , Harper RM . Brain structural changes in obstructive sleep apnea . Sleep. 2008. ; 31 ( 7 ): 967 – 977 . [PMC free article] [PubMed] [Google Scholar]
- 27. Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force . Sleep. 1999. ; 22 ( 5 ): 667 – 689 . [PubMed] [Google Scholar]
- 28. Veasey SC . Obstructive sleep apnea: re-evaluating our index of severity . Sleep Med. 2006. ; 7 ( 1 ): 5 – 6 . [DOI] [PubMed] [Google Scholar]
- 29. Lopez-Jimenez F , Somers VK . Stress measures linking sleep apnea, hypertension and diabetes–AHI vs arousals vs hypoxemia . Sleep. 2006. ; 29 ( 6 ): 743 – 744 . [DOI] [PubMed] [Google Scholar]
- 30. Lanfranchi P , Somers VK . Obstructive sleep apnea and vascular disease . Respir Res. 2001. ; 2 ( 6 ): 315 – 319 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mediano O , Barceló A , de la Peña M , Gozal D , Agusti A , Barbé F . Daytime sleepiness and polysomnographic variables in sleep apnoea patients [Portuguese] . Rev Port Pneumol. 2007. ; 13 ( 6 ): 896 – 898 . [DOI] [PubMed] [Google Scholar]
- 32. Baguet JP , Hammer L , Lévy P , et al . The severity of oxygen desaturation is predictive of carotid wall thickening and plaque occurrence . Chest. 2005. ; 128 ( 5 ): 3407 – 3412 . [DOI] [PubMed] [Google Scholar]
- 33. Asano K , Takata Y , Usui Y , et al . New index for analysis of polysomnography, ‘integrated area of desaturation’, is associated with high cardiovascular risk in patients with mild to moderate obstructive sleep apnea . Respiration. 2009. ; 78 ( 3 ): 278 – 284 . 1.1159/000202980 [DOI] [PubMed] [Google Scholar]
- 34. Chen HL , Lu CH , Lin HC , et al . White matter damage and systemic inflammation in obstructive sleep apnea . Sleep. 2015. ; 38 ( 3 ): 361 – 370 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tummala S , Roy B , Park B , et al . Associations between brain white matter integrity and disease severity in obstructive sleep apnea . J Neurosci Res. 2016. ; 94 ( 10 ): 915 – 923 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bunce D , Anstey KJ , Cherbuin N , et al . Cognitive deficits are associated with frontal and temporal lobe white matter lesions in middle-aged adults living in the community . PLoS One. 2010. ; 5 ( 10 ): e13567 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jones K , Harrison Y . Frontal lobe function, sleep loss and fragmented sleep . Sleep Med Rev. 2001. ; 5 ( 6 ): 463 – 475 . [DOI] [PubMed] [Google Scholar]
- 38. Karnath HO , Baier B , Nägele T . Awareness of the functioning of one’s own limbs mediated by the insular cortex? J Neurosci. 2005. ; 25 ( 31 ): 7134 – 7138 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Oppenheimer S , Cechetto D . The insular cortex and the regulation of cardiac function . Compr Physiol. 2016. ; 6 ( 2 ): 1081 – 1133 . [DOI] [PubMed] [Google Scholar]
- 40. Yadav SK , Kumar R , Macey PM , Woo MA , Yan-Go FL , Harper RM . Insular cortex metabolite changes in obstructive sleep apnea . Sleep. 2014. ; 37 ( 5 ): 951 – 958 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhang ZH , Dougherty PM , Oppenheimer SM . Monkey insular cortex neurons respond to baroreceptive and somatosensory convergent inputs . Neuroscience. 1999. ; 94 ( 2 ): 351 – 360 . [DOI] [PubMed] [Google Scholar]
- 42. Gozal E , Row BW , Schurr A , Gozal D . Developmental differences in cortical and hippocampal vulnerability to intermittent hypoxia in the rat . Neurosci Lett. 2001. ; 305 ( 3 ): 197 – 201 . [DOI] [PubMed] [Google Scholar]
- 43. Pae EK , Chien P , Harper RM . Intermittent hypoxia damages cerebellar cortex and deep nuclei . Neurosci Lett. 2005. ; 375 ( 2 ): 123 – 128 . [DOI] [PubMed] [Google Scholar]