Fig. 3.
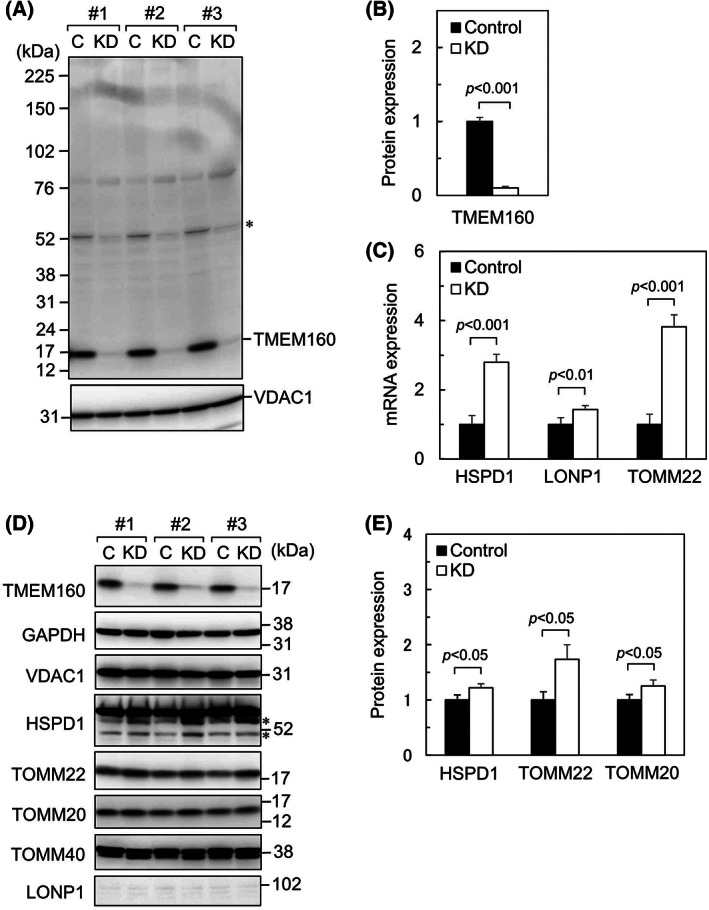
Depletion of TMEM160 led to induction of expression of the UPRmt‐related mitochondrial molecular chaperone HSPD1 and mitochondrial protein import receptors TOMM22 and TOMM20. HepG2 cells were transfected with control siRNA and TMEM160‐targeted siRNA. An asterisk indicates an unknown band that appeared at approximately 52 kDa; (A) proteins extracted from the cells were subjected to immunoblot analysis to determine TMEM160 protein levels (n = 3) [C, control cell lysate; KD, TMEM160‐depleted cell lysate]. (B) Protein expression of TMEM160 was quantified. data were analyzed using Student's t‐test, and the error bars represent mean ± standard deviation (SD). Protein expression levels of the control were set at 1. (C) mRNA levels of HSPD1, LONP1, and TOMM22 were examined using quantitative PCR (n = 4). Data were analyzed using Student's t‐test, and the error bars represent mean ± SD. mRNA levels of the control were set as 1 [HSPD1, heat shock protein family D (Hsp60) member 1; LONP1, Lon peptidase 1; TOMM22, translocase of outer mitochondrial membrane 22]. (D) the proteins extracted from cells were subjected to immunoblot analysis to determine expression levels of the indicated proteins (n = 3). Asterisks indicate bands corresponding to hypothetical degraded portions of HSPD1 [GAPDH, glyceraldehyde‐3‐phosphate dehydrogenase; TOMM40 and TOMM20, translocase of outer mitochondrial membrane 40 and 20, respectively]. (E) Protein expression of HSPD1, TOMM22, and TOMM20 was quantified. data were analyzed using Student's t‐test, and the error bars represent mean ± SD. Protein expression levels of the control were set at 1.
