Fig. 3. DDB1 and next-generation CELMoD agents further poise CRBN substrates for ubiquitination in disease contexts.
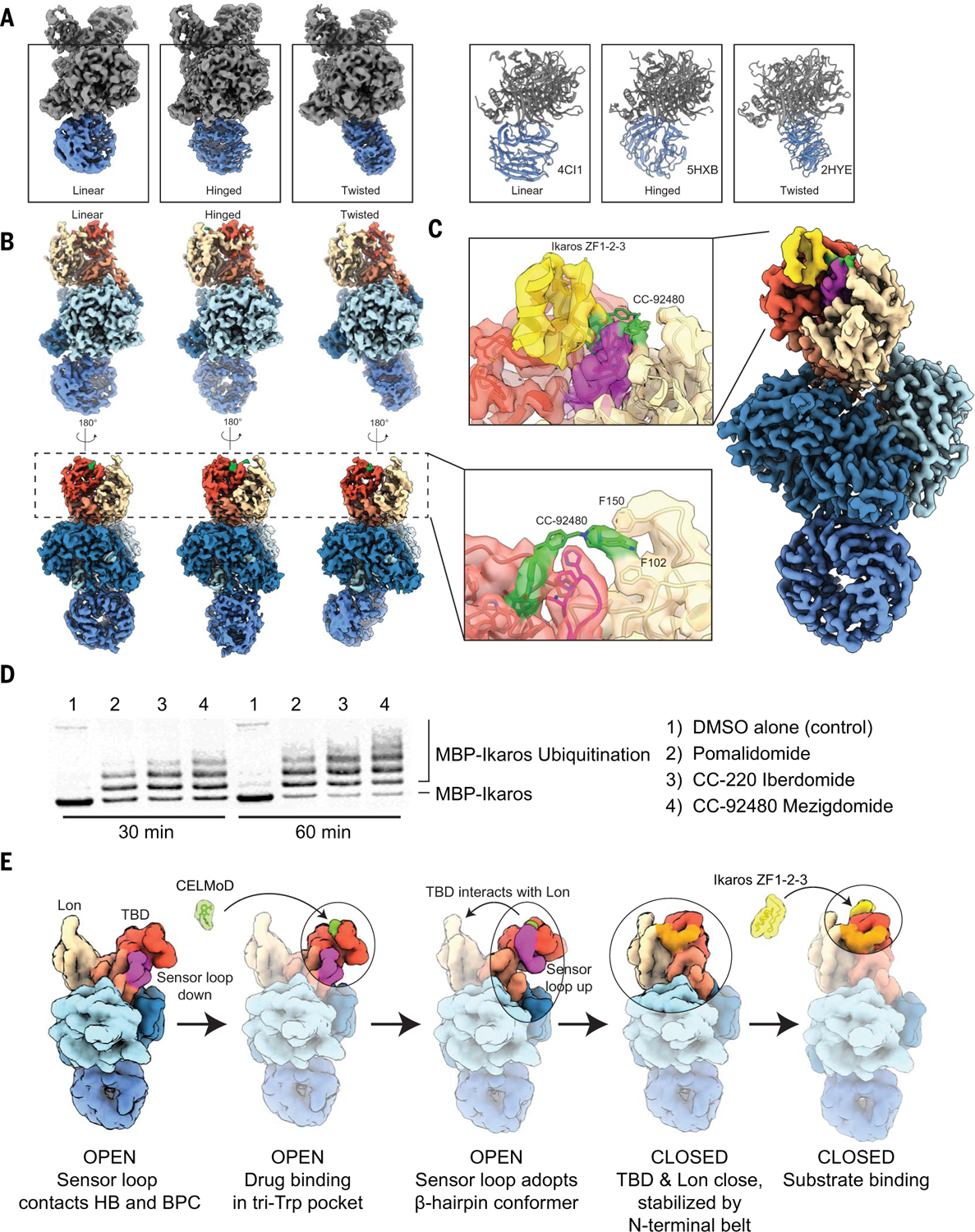
(A) Three-dimensional classification of unliganded CRBN-DDB1 yields three discrete positions of DDB1’s mobile BPB propeller (colored blue). (Left) ~3.5-Å-resolution cryo-EM reconstructions of CRBN-DDB1 with BPB in a linear (left), hinged (middle), or twisted (right) position. (Right) Structural models in similar orientations for reference, adopting a linear (PDB ID 4CI1, left), hinged (PDB ID 5HXB, middle), or twisted (PDB ID 2HYE, right) position. (B) The ~3.2-Å-resolution cryo-EM reconstructions of CRBN-DDB1 complexed with mezigdomide with a linear (left), hinged (middle), or twisted (right) position of BPB. (Inset) ~3.1-Å-resolution focused refinement of particles from all three orientations reveal a connection between mezigdomide and the Lon domain, “stapling” the CRBNclosed conformation. (C) The ~3.1-Å-resolution reconstruction of mezigdomide-induced CRBNclosed bound to Ikaros ZF1-ZF2-ZF3. The inset shows a focused view of connection between mezigdomide and the Lon domain, stapling CRBNclosed in the presence of Ikaros. (D) In vitro ubiquitination assay tracking MBP-Ikaros ubiquitination in the presence of different molecules used in this study (labeled on the right). (E) Mechanistic model illustrating pathway of assembly. First, unliganded CRBN is open with the sensor loop attached to HB. Second, ligand is added and associates with TBD in the hydrophobic pocket. Third, binding of ligand in the tri-Trp pocket stabilizes the sensor loop as a β-hairpin detached from the HB. Fourth, sensor loop refolding promotes transition of CRBNopen to CRBNclosed without an observed intermediate, and the N-terminal belt is seated (orange). Fifth, substrate is recruited to the CRBNclosed conformation for subsequent ubiquitination by CRL4.
