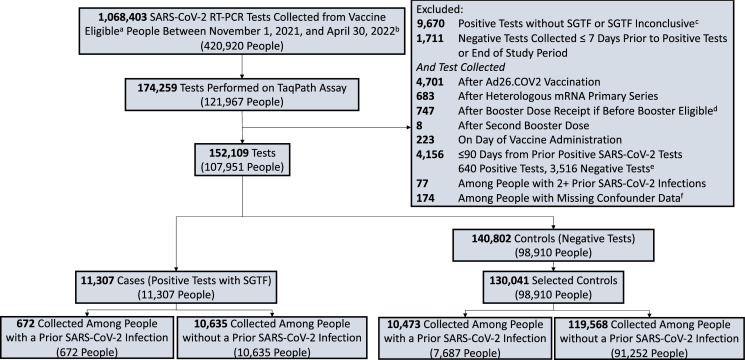
Fig 2. Selection of tests for the case–control analysis.
The sample was limited to RT-PCRs run on the TaqPath COVID-19 (Thermo Fisher Scientific) assay among vaccine-eligible individuals. Case status was defined based on the reflex results. We included all positive tests (cases) and up to three negative tests (controls) per person. Cases and controls were stratified by presence of a documented prior infection (a positive RT-PCR or rapid antigen test at least 90 days before the included test). aVaccine eligibility was defined as age ≥5 years. bThe first SGTF defined Omicron (BA.1 lineage) variant infection in the study population was identified on November 11, 2021. cSamples with a positive reflex RT-PCR but that did not meet our SGTF definition (an ORF1ab Ct value of <30 and S-gene Ct–ORF1ab Ct value ≥5; or 2] ORF1ab Ct value <30 and S-gene Ct value ≥40. dExcluded tests that were performed after a person was given a booster dose before FDA authorization or that was given less than 150 days after primary vaccination completion. eThe median time between the 640 dropped positives and the prior positive was 5 days (first-third quartiles: 3 to 7 days). fThere were 134 people with missing SVI data, 40 people with missing sex data and none with missing age data. People were allowed to contribute up to three negative tests to the control sample. If they had more than three negative tests over the study period, three tests were randomly selected. If a person had more than one negative test within a 7-day period, one test performed within that period was randomly selected. COVID-19, Coronavirus Disease 2019; RT-PCR, reverse transcription PCR; SGTF, S-gene target failure.