FIGURE 1.
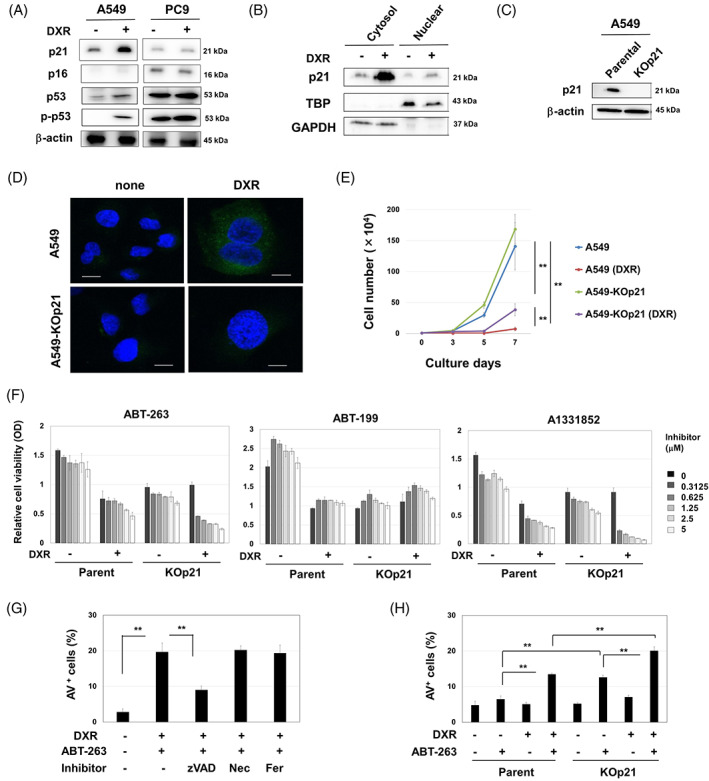
Caspase‐dependent senolysis by ABT‐263 in DXR‐treated A549‐KOp21 cells. (A) Cancer cells were treated with DXR (0.1 μM) for 48 h. Thereafter, immunoblotting was performed. β‐Actin was used as a control. (B) After treating with DXR (0.1 μM) for 48 h, the cytoplasmic and nuclear fractions from A549 cells were separated, and immunoblotting was performed. TBP and GAPDH were used as controls for nuclear and cytoplasmic proteins, respectively. (C) Immunoblot of parental A549 and KO‐p21 cells. (D) Parental A549 and KO‐p21 cells were treated with DXR (0.1 μM) for 48 h and stained with SPiDER β‐gal. Confocal imaging reveals nuclei (blue) and SPiDER β‐gal (green). Scale bar: 10 μm. (E) After treating with DXR (0.1 μM) for 48 h, the growth of A549 and A549‐KOp21 cells was examined in triplicate. **p < 0.01. (F) A549 cells and A549‐KOp21 cells were cultured with or without DXR (0.1 μM) for 48 h. After removing the medium, the cells were cultured with the indicated inhibitors for 48 h. Thereafter, cell viability was determined. Data are the means ± SD of three replicates. (G) After the treatment with DXR (0.1 μM) for 48 h, A549 cells were cultured with ABT‐263 (2.5 μM) with the indicated inhibitors (10 μM) for 48 h. Thereafter, cell apoptosis was measured by flow cytometry. Data are the means ± SD of three replicates. **p < 0.01. (H) Similarly, A549 and A549‐KOp21 cells were cultured with or without DXR (0.1 μM) for 48 h. After harvesting, the cells were cultured with ABT‐263 (2.5 μM) for 48 h, and an apoptosis assay was performed. Data are the means ± SD of three replicates. **p < 0.01. DXR, doxorubicin; Fer, ferrostatin‐1; Nec, necrostatin‐1.
