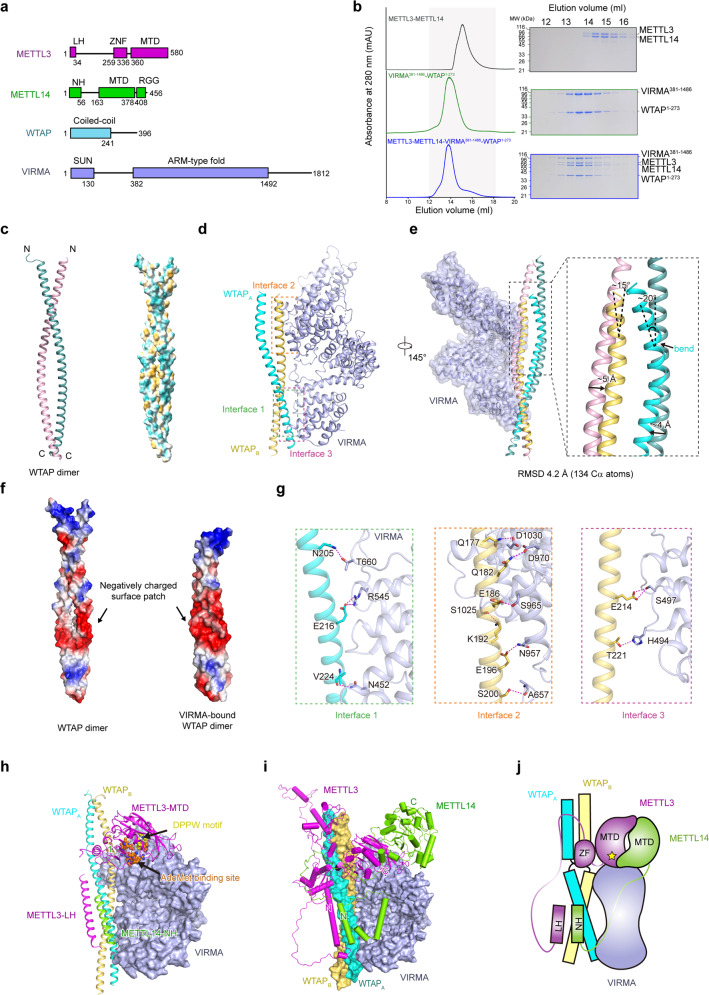
Fig. 1. Structure of the human m6A writer complex.
a Schematic diagram of the domain information of METTL3, METTL14, WTAP and VIRMA. LH leader helix, ZFD zinc finger domain, MTD MTase domain, NH N-terminus helix. b Gel filtration analysis of the human METTL3–METTL14–WTAP1–273–VIRMA381–1486 complex. c The crystal structure of WTAP alone. Two molecules of WTAP form a symmetric parallel α-helical coiled-coil, with the two chains colored light pink and light teal, respectively. d The cryo-EM structure of WTAP–VIRMA complex in the METTL3–METTL14–WTAP1–273–VIRMA381–1486 quaternary complex. Three interaction interfaces can be observed between WTAPs and VIRMA. WTAPA, cyan; WTAPB, orange. e Comparison of the crystal structure of WTAP alone and the cryo-EM structure of VIRMA-bound WTAPs. VIRMA-bound WTAPs bend around the observed N-termini. f Electrostatic surfaces of WTAP alone (PDB: 7YFJ) and VIRMA-bound WTAPs (PDB: 7YG4). g Details of the observed interaction interfaces between WTAP and VIRMA. h, i Structural model of the METTL3–METTL141–399–WTAP148–237–VIRMA342–1292 complex based on CXMS measurement and AI prediction. METTL3 and METTL14 are shown in green and magenta, respectively. METTL3 LH is docked close to WTAP, and the MTC core catalytic center is docked close to VIRMA, with the DPPW motif and AdoMet binding site positioned towards a cleft between WTAP and VIRMA. j Schematic diagram of the modeled METTL3–METTL141–399–WTAP148–237–VIRMA342–1292 complex.