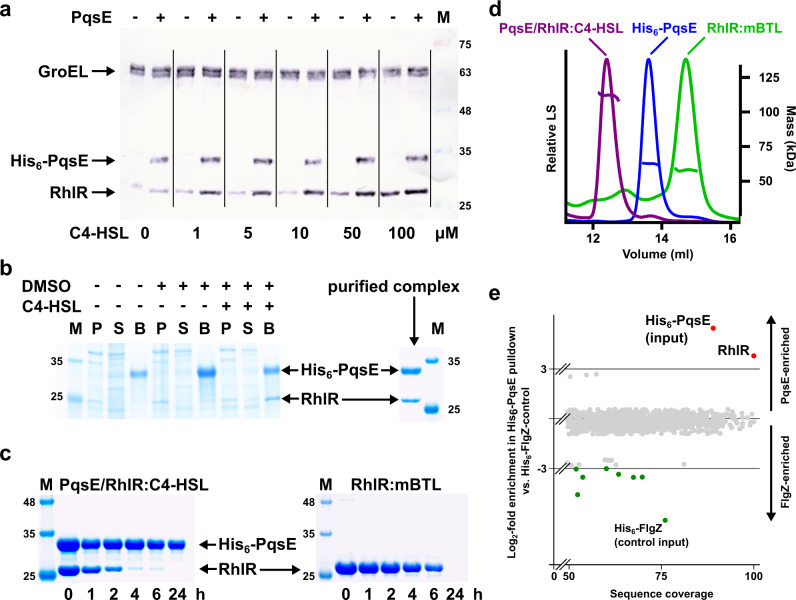
Fig. 2. Interaction of RhlR and PqsE.
a Western blot demonstrating the synergistic solubility-enhancing effect of C4-HSL and PqsE on RhlR. RhlR and His6-PqsE were expressed from plasmid vectors in E. coli BL21-CodonPlus(DE3)-RIL in the presence of the indicated concentrations of C4-HSL. Soluble extracts were analyzed using GroEL as a control. The marker (M) indicates the molecular weight in kDa. A typical result from at least three independent experiments is shown. The corresponding whole-cell extracts are shown in Supplementary Fig. 1a. b SDS-PAGE of a co-expression analysis of His6-tagged PqsE and RhlR in E. coli BL21-CodonPlus(DE3)-RIL. Induced cells were harvested, lysed and separated into an insoluble (P) and a soluble fraction (S), which was used to incubate Ni-NTA beads, washed and then boiled in SDS-loading buffer (B). The marker (M) indicates the molecular weight in kDa. A typical result from at least three independent experiments is shown. c Time-dependent stability analysis of PqsE/RhlR:C4-HSL and RhlR:mBTL. Purified proteins were incubated at 37 °C and the soluble fraction was analyzed by SDS-PAGE after the indicated period. The marker (M) indicates the molecular weight in kDa. A typical result from at least three independent experiments is shown. d SEC-MALS analysis of RhlR purified in the presence of mBTL, of PqsE and of the PqsE/RhlR:C4-HSL complex. The molecular mass of PqsE suggests that the protein is dimeric. PqsE and RhlR interact in a 2:2 complex. e Proteomic analysis of a pulldown with His6-tagged PqsE in P. aeruginosa PA14 vs. a His6-FlgZ control. Buffers were supplemented with 50 µM C4-HSL. Proteins with more than 50% sequence coverage in the analysis are shown. Log2-fold enrichments larger than 3 (Ni-NTA bead eluate vs. whole-cell extract) are colored in red and green, respectively. The analysis confirms the interaction of PqsE and RhlR in P. aeruginosa. Source data are provided as a Source Data file.