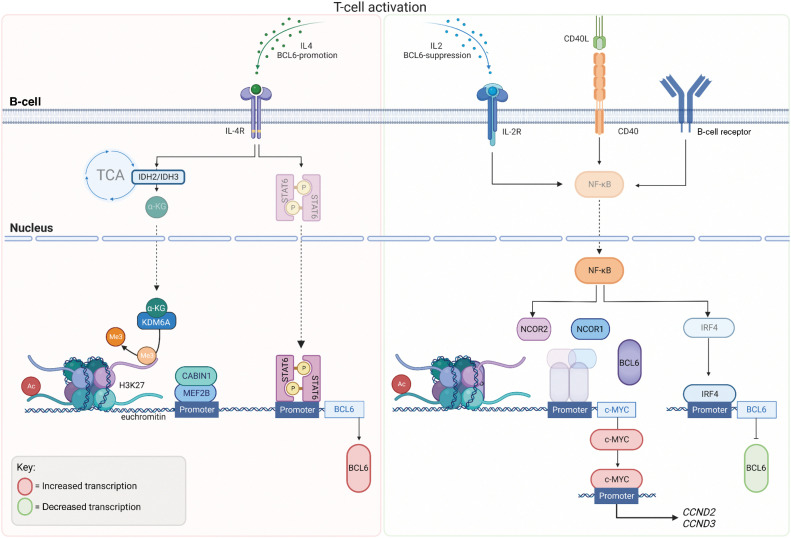
Figure 3.
BCL6 Regulation: BCL6 expression in B cells during GC formation occurs once B cells have matured with correct antigen affinity. T-cell activation and IL4 stimulation via cytokines drives metabolic tricarboxylic acid (TCA) cycle reprogramming through IDH2 and IDH3 and hence the production of alpha ketoglutarate (αKG), which acts as a substrate for Lysine-specific demethylase 6A (KDM6A) leading the loss of repressive H3K27me3 marks. KDM6A is recruited by phosphorylated Signal transducer and activator of transcription (STAT6), to the BCL6 locus causing the demethylation of H3K27me3, leading to euchromatin and active transcription of BCL6. Myocyte enhancer-binding factor 2B (MEF2B), the master regulator of BCL6 expression is regulated via its corepressor phosphatase, CABIN 1 (Calcineurin Binding Protein 1) and binds to BCL6 promoter, following T-cell activation. MEF2B binds predominantly to chromatin with histone marks, H3K4me3 (trimethylation, Me3) and H3K27ac (acetylation, Ac) suggestive of active marks. Following IL2 stimulation, CD40 activation and B-cell receptor activation, NF-kB is translocated to the nucleus where it suppresses the formation of the BCL6–NCOR complex, alleviating the negative regulation of the c-MYC promoter by BCL6. NF-kB activation promotes IRF4 to bind to the BCL6 promoter to negative regulate its transcription. (Created with BioRender.com)