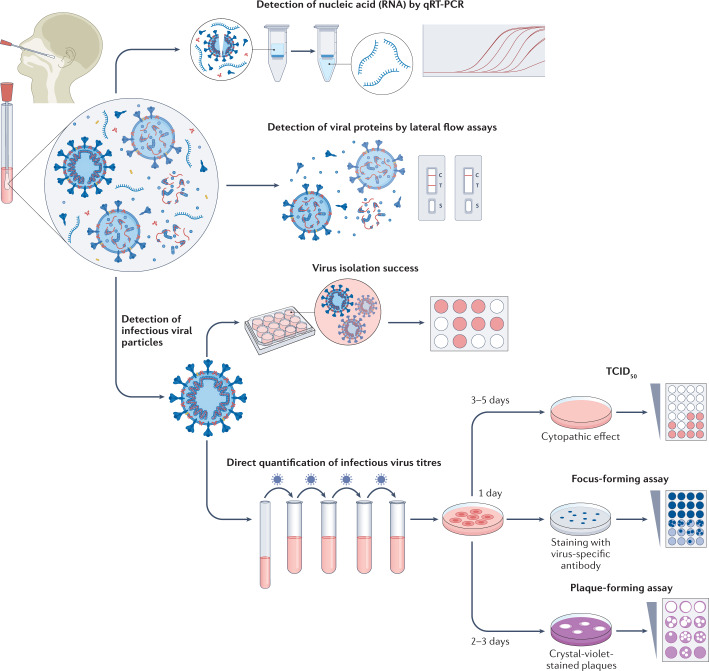
Fig. 1. Methods to measure infectious virus and RNA viral load.
Swab specimens from the nasopharynx or oropharynx are used for detection of SARS-CoV-2 viral loads. Detection of viral nucleic acids (RNA) is performed by quantitative real-time PCR (qRT-PCR). Viral RNA is extracted from lysed virus, reverse transcribed and amplified by qPCR using primers specific for one or more target regions in the viral genome. The amplification cycle at which samples cross the threshold (cycle threshold) defines the amount of viral RNA. RNA viral load can be expressed as the number of viral RNA copies per millilitre, or by the arbitrary test-specific cycle threshold value. Lateral flow assays detect the presence of specific viral proteins in the lysed viral particles. SARS-CoV-2 nucleocapsid is used in most antigen-detecting (rapid) diagnostic tests. The presence of infectious (replication-competent) virus in respiratory specimens can only be determined by the recovery of virus in cell culture by isolation or by quantification of infectious virus titres using 50% tissue culture infectious dose (TCID50), focus-forming assays or plaque-forming assays. Virus isolation is performed by applying infectious medium on the monolayer of cells; isolation success is determined by the presence of a cytopathic effect approximately 3–5 days post-infection. White colour indicates the presence of a cytopathic effect in cells. For quantification of infectious virus titres, serial dilutions of respiratory samples are performed and used for inoculation on the monolayer of cells. In TCID50, 3–5 days post-infection, viral-induced cytopathic effect is classically defined using microscopy. In focus-forming assays, cells are fixed 1 day post-infection and immunostaining with virus-specific antibodies is performed to detect groups of infected cells (foci). The foci, indicating the presence of infectious virus, are displayed in blue. In plaque-forming assays, plates are fixed 2–3 days post-infection and stained with crystal violet; wells with individual plaques are used to determine viral titres. The plaques, indicating the presence of infectious virus, are displayed in white.