Figure 1. aPKCs have a contextual oncogene or tumor suppressor role.
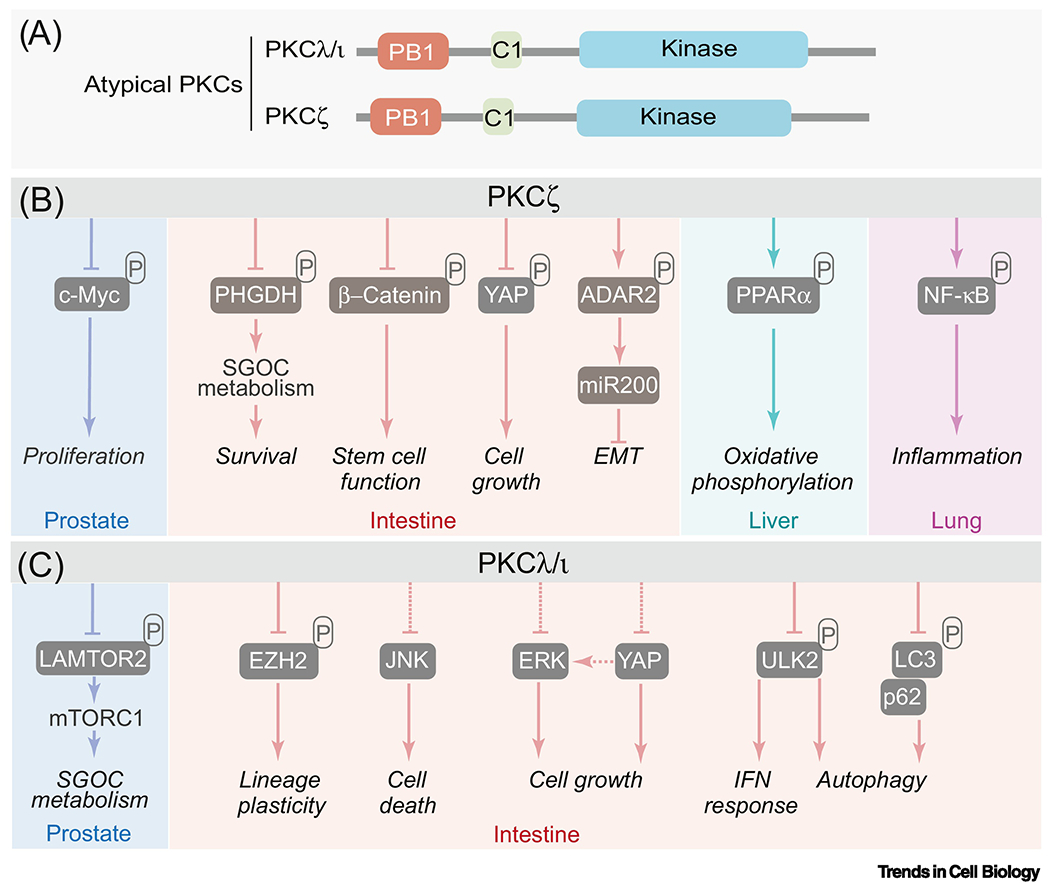
(A) The atypical protein kinase C subfamily is constituted by two members: PKCζ and PKCλ/ι. The aPKCs differ from the other members of the PKC family in that they lack functional C2 and the C1 domain does not bind diacylglycerol. The regulatory domain of the aPKCs also harbor a PB1 domain, a highly conserved protein interaction module that regulates their binding to other PB1-containing proteins such as the autophagy receptors and signaling adaptor p62.
(B-C) PKCζ (B) and PKCλ/ι (C) and their direct phosphorylation substrates and binding partners. The aPKCs have a contextual oncogene or tumor suppressor role in different types of cancer. They have pleiotropic biological functions mediated by phosphorylation of substrates or binding to selective interactors. SGOC: serine glycine one-carbon; EMT: epithelial mesenchymal transition; IFN: interferon.
