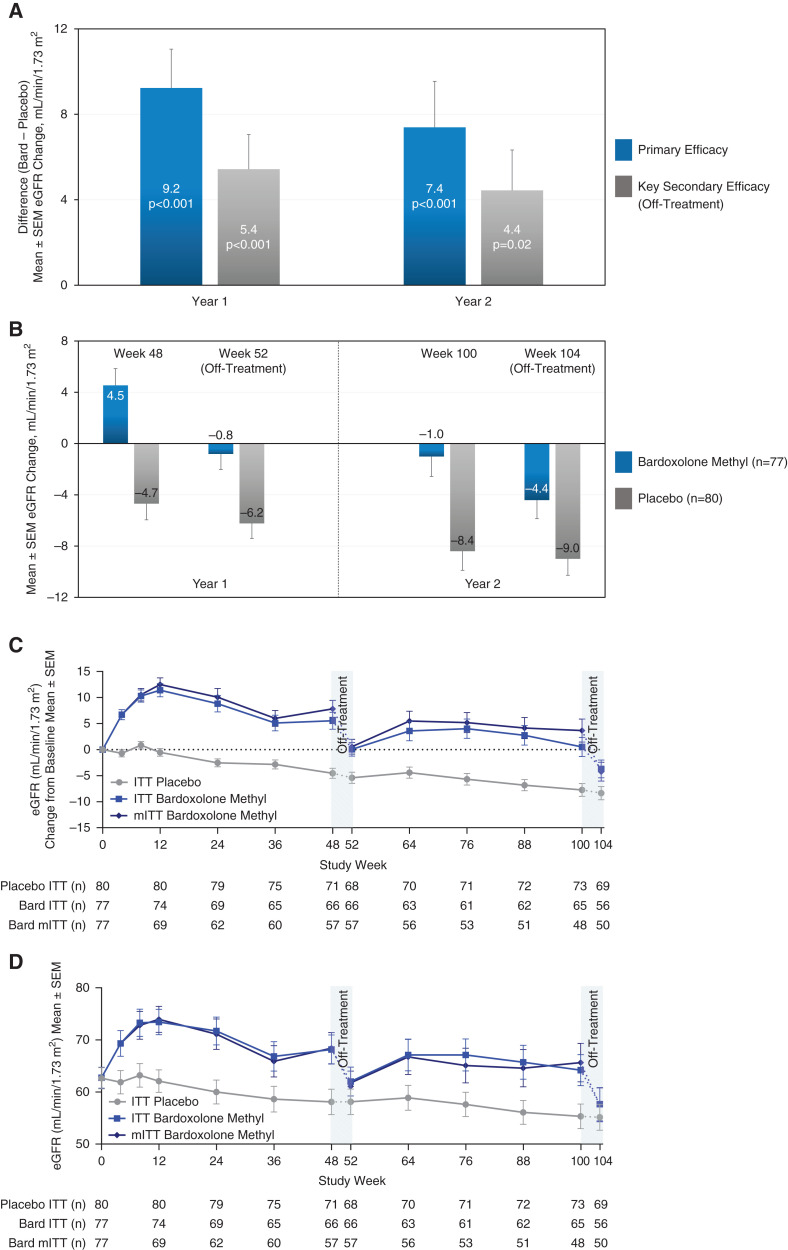
Figure 1.
Primary and key secondary efficacy results from CARDINAL (intention-to-treat population). (A) Mean difference between treatment groups for the primary end point, changes from baseline in eGFR at 48 weeks (year 1) and 100 weeks (year 2), and for the key secondary end point, off-treatment changes from baseline in eGFR at 52 weeks (year 1) and 104 weeks (year 2). The primary end point was analyzed using mixed model repeated measures. The model included all available eGFR values collected through week 100 (excluding week 52) for the intention-to-treat (ITT) population (n=157, with n=80 for placebo and n=77 for bardoxolone methyl). eGFR data were available for 71 patients randomized to placebo and 66 patients randomized to bardoxolone methyl (bard) at 48 weeks, and for 73 patients randomized to placebo and 65 patients randomized to bardoxolone methyl at 100 weeks, and missing data were not imputed. We assessed key secondary end points 4 weeks after last dose in year 1 at 52 weeks and in year 2 at 104 weeks and analyzed using analysis of covariance for the ITT population (n=157, with n=80 for placebo and n=77 for bardoxolone methyl). Off-treatment eGFR data (collected 4 weeks after last dose) were available for 68 patients randomized to placebo and 66 patients randomized to bardoxolone methyl at 52 weeks, and for 69 patients randomized to placebo and 56 patients randomized to bardoxolone methyl at 104 weeks. For the key secondary end points, we imputed missing data using multiple imputation on the basis of the randomized treatment group. (B) Mean changes from baseline in patients randomized to bardoxolone methyl (n=77) and placebo (n=80) contributing to primary (at 48 and 100 weeks) and key secondary (at 52 and 104 weeks) efficacy analyses. (C) Observed mean (±SEM) change from year 1 baseline (i.e., before starting intervention) in eGFR for the ITT population and the modified ITT population (mITT) through the 104 weeks of the study. The mITT analysis assesses the effect of receiving study drug in the ITT population and excludes any eGFR values collected after final dose (detailed in Supplemental Table 3). Off-treatment periods are represented by the dash and only include eGFR data collected 4 weeks after last dose. Additional eGFR values, collected approximately 104 weeks after randomization, irrespective of time off study drug, were available for a total of 78 patients randomized to placebo and 72 patients randomized to bardoxolone methyl (Table 2). (D) Observed mean (±SEM) eGFR values for the ITT population and the mITT population through the 104 weeks of the study. The mITT analysis assesses the effect of receiving study drug in the ITT population and excludes any eGFR values collected after final dose. Off-treatment periods are represented by the dash.