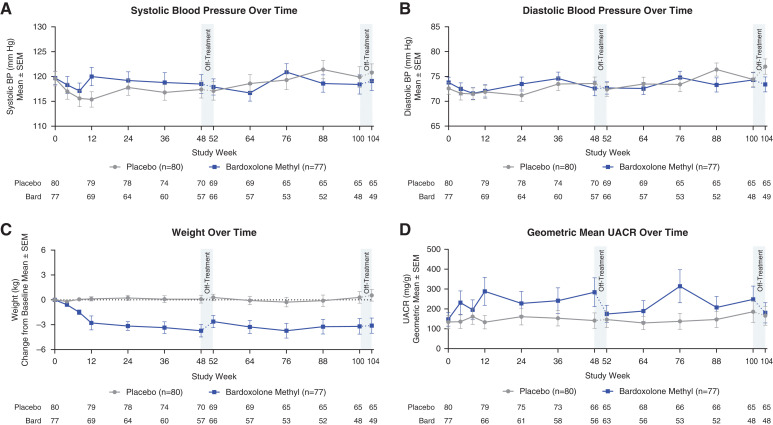
Figure 3.
Safety parameters over time. (A and B) Mean (±SEM) systolic and diastolic BP for patients randomized to bardoxolone methyl (bard; n=77) or placebo (n=80) through the 104 weeks of the study. Data collected during the on-treatment period are represented by the solid line, and off-treatment data are represented by the dashed line. Mean values at 52 and 104 weeks include data collected 28 days after last dose for patients that discontinued early in the first or second year of treatment, respectively. (C) Mean (±SEM) change from baseline in weight for patients randomized to bardoxolone methyl (n=77) and patients randomized placebo (n=80) through 104 weeks. Data collected during the on-treatment period are represented by the solid line, and off-treatment data are represented by the dashed line. Mean values at 52 and 104 weeks include data collected 28 days after last dose for patients that discontinued early in the first or second year of treatment, respectively. (D) Geometric mean (±SEM) urinary albumin-creatinine ratio (UACR) for patients randomized to bardoxolone methyl (n=77) and patients randomized to placebo (n=80) through 104 weeks. Data collected during the on-treatment period are represented by the solid line, and off-treatment data are represented by the interrupted line. Mean values at 52 and 104 weeks include data collected 28 days after last dose for patients that discontinued early in the first or second year of treatment, respectively.