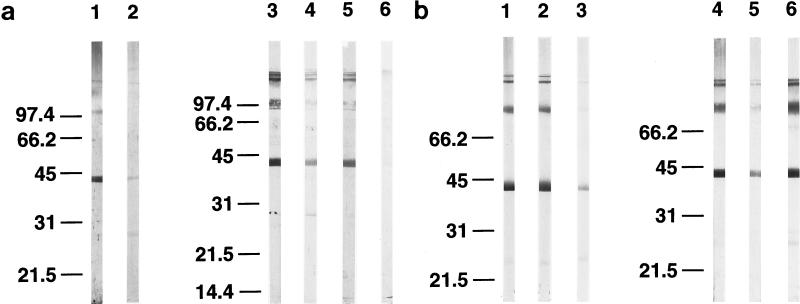
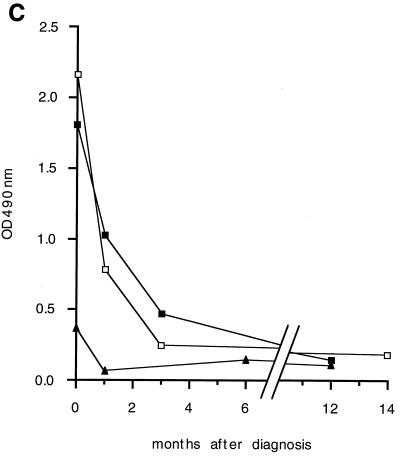
FIG. 3.
Reactivity of VL patient sera against recombinant papLe22. (a) GST-papLe22 was purified and electrophoresed as described in the legend for Fig. 2a. One microgram of protein was loaded per centimeter of gel. Western blotting was performed with sera, diluted 1:50, from patient 1 at diagnosis (lanes 1 and 3) and 14 months later (lane 4) and from patient 2 at diagnosis (lanes 2 and 5) and 12 months later (lane 6). Anti-IgM (lanes 1 and 2) and anti-IgG (lanes 3 to 6) peroxidase-conjugated secondary antibodies were diluted 1:100 and 1:1,000, respectively. (b) Diagnostic sera from patient 1 (lanes 1 to 3) and patient 3 (lanes 4 to 6) were incubated as described for panel a. Anti-IgG1 (lanes 1 and 4), anti-IgG2 (lanes 2 and 5), and anti-IgG3 (lanes 3 and 6) peroxidase-conjugated secondary antibodies were diluted 1:100. Molecular mass markers in kilodaltons are indicated. (c) GST-papLe22 and GST were purified as described in the legend for Fig. 2a and were eluted from glutathione-Sepharose with 0.1% SDS in PBS. Recombinant proteins at 0.05 μg per well were used to coat microtiter plates and were sequentially incubated with patient serum diluted 1:100 and peroxidase-labeled anti-human IgG. Absorbances obtained with GST-papLe22 were corrected for absorbances measured with GST alone. Data for diagnosis and follow-up sera from patient 1 (open squares), patient 2 (triangles), and patient 4 (solid squares) are shown. Sera from LST-negative controls and positive asymptomatic subjects gave values for optical density at 490 nm (OD490 nm) below 0.12.