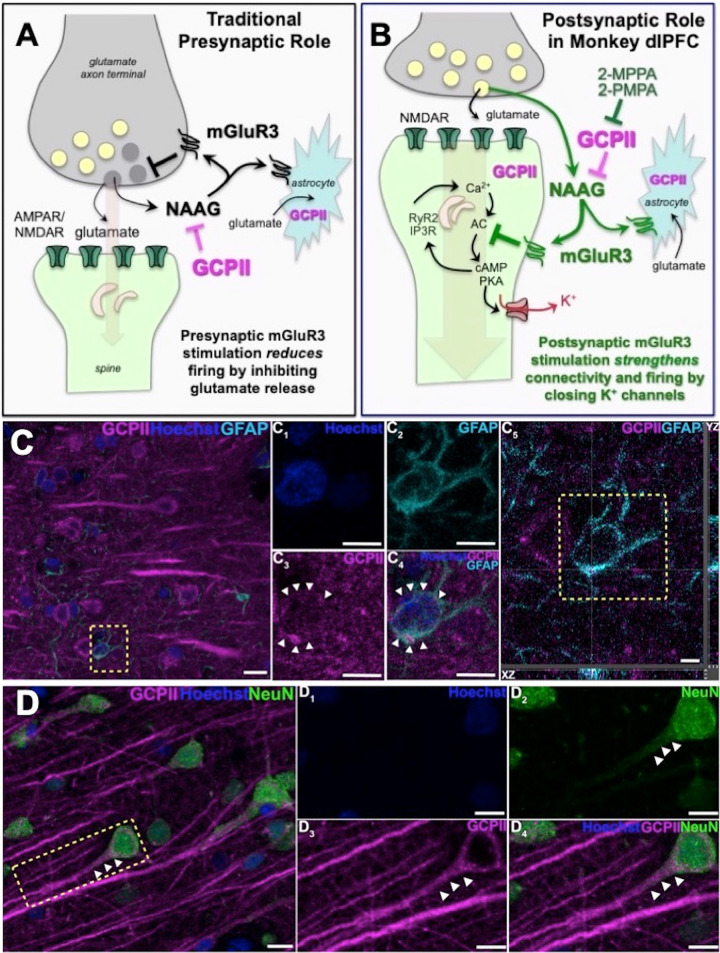
Fig. 1. GCPII-NAAG-mGluR3 signaling in primate dlPFC.
A Traditional view of mGluR3 signaling in a glutamate synapse, where receptors are predominately on presynaptic terminals and on astrocytes, where they reduce glutamate signaling by inhibiting release and increase uptake, respectively. NAAG is co-released with glutamate, and is selective for mGluR3; GCPII catabolism of NAAG would increase neuronal firing in these traditional circuits. B GCPII-NAAG-mGluR3 signaling in layer III of the primate dlPFC, where mGluR3 are not presynaptic, but rather are post-synaptic on spines, and NAAG stimulation of mGluR3 inhibits cAMP-K+ channel signaling to increase synaptic efficacy and enhance neuronal firing. Thus, GCPII catabolism of NAAG decreases neuronal firing in these recently evolved circuits. C Multiple label immunofluorescence showing GCPII labeling (magenta) in its traditional localization in astrocytes which are co-labeled with GFAP (cyan); Hoescht (blue) labels nuclei of all cells. One representative astrocyte is outlined by the yellow dashed box in (C. C1-C4) correspond to the boxed area in (C). In (C1–C4) GCPII labeling clearly outlines the GFAP positive astrocyte soma, as demarcated by the white arrowheads, (C5) Orthogonal sectioning of this region (corresponding region of interest from C outlined by the yellow box). Selected Z-stack image shows co-localization of GCPII and GFAP (magenta and cyan) across three different planes for one point, as indicated by the crossed dashed lines. The right-side bar demonstrates labeling in the YZ plane, while the bottom bar represents labeling in the XZ plane. D GCPII is also co-localized in neurons (NeuN, green) with a pyramidal cell-like morphology. White arrowheads highlight GCPII labeling in an apical dendrite. Scale bars: (C,D) 10 μm; (C1-C4) 5 μm; (C5) 2.5 μm; (D1-D4) 10 μm.