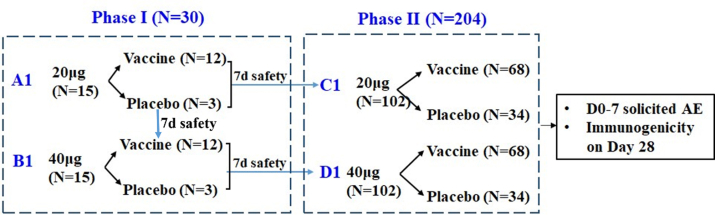
Supplementary Figure S1.
Trial procedure. 30 and 205 participants were eligible and randomized into the phase I and phase II trials respectively. During the phase 1 trial, participants in each cohort were randomly assigned in a 4:1 ratio to receive an intramuscular injection of SCTV01C (20 μg or 40 μg) or saline placebo; whereas participants were assigned in a 2:1 ratio for each cohort of the phase 2 trial. The enrollment started with cohort A1. Upon completing 7 days safety observation, enrollment began in cohort B1 and cohort C1 simultaneously. Following 7 days safety observation of cohort B1, enrollment of Cohort D1 was initiated.