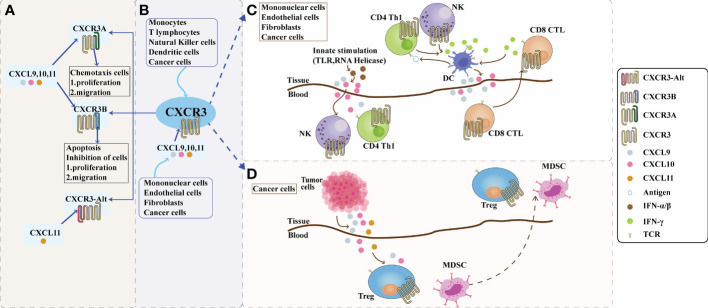
Figure 1.
The role of CXCR3 and its ligand pathway in the tumor microenvironment. (A): Composition of CXCR3. Depending on the piece of the amino terminus of the receptor, CXCR3 can be divided into three splice variants: CXCR3-A, CXCR3-B, and a truncated variant CXCR3-alt. CXCR3-A and CXCR3-B have ligands CXCL9, CXCL10, and CXCL11, while CXCR3-alt binds only CXCL11. CXCR3A promotes cell proliferation and migration in the tumor microenvironment, while CXCR3B inhibits cell proliferation and migration. (B): The CXCL9,10,11/CXCR3 axis in the tumor microenvironment. CXCL9, 10, and 11 are mainly secreted by monocytes, endothelial cells, fibroblasts, and cancer cells in response to IFN-γ. The CXCL9,10,11/CXCR3 axis works in two main directions; paracrine signals for immune activation and autocrine signals for cancer cell proliferation and metastasis. (C): Paracrine secretion of CXCR3 is also known as innate immune activation. This axis is primarily used for migration, differentiation, and activation of immune cells for paracrine signaling. Immunoreactivity occurs through this axis by recruiting CTLs, NK cells, and macrophages. Activating TLRs and RNA helicases leads to the release of IFN-a/β from histiocytes and endothelial cells, followed by the secretion of chemokines such as CXCL10; CXCL10 recruits NK and CD4+ Th1 cells into the target tissue, while CD4+ Th1 cells release IFN-γ in an antigen-specific manner. DCs and other resident cells in the tissue take up IFN-γ and induce the production and secretion of CXCL9 and CXCL10; secreted CXCL9 and CXCL10 recruit CD8+ CTL into the tissue. IFN-γ produced by CD8+ CTL further stimulates tissue cells to produce more CXCL9 and CXCLI0. Increased chemokine release and inflammatory responses are amplified, leading to further recruitment of CXCR3-expressing Th1 and CD8+ TIL cells. Figure (D): The autocrine function of CXCR3. In terms of autocrine signaling, cancer cells are predisposed to metastasis due to the activity of tumor-derived ligands, mainly through CXCR3A. Tumor-derived chemokines are also responsible for the recruitment of Th cells, Tregs, and MDSCs, which play a role in creating a tumor-friendly microenvironment. CTLs, cytotoxic lymphocytes; NK, natural killer cells; MDSCs, myeloid-derived suppressor cells; Th1, T helper 1; Tregs, regulatory T cells.