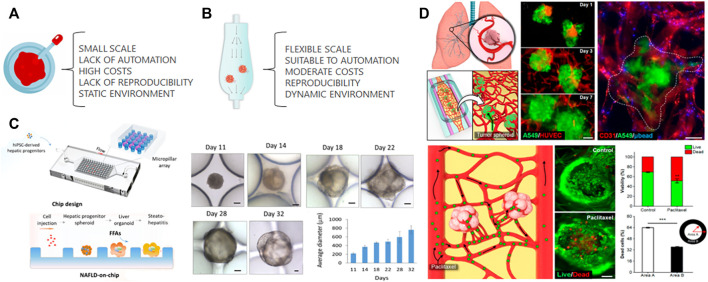
FIGURE 1.
Organoids culture. (A) Traditional organoid culture relies on several disadvantages that can be partially solved by culturing organoids in a microfluidic chip (B). The laminar flow of microfluidics provides a controlled dynamic environment increasing the reproducibility while reducing cost of organoid culture. The large scale can be reached by parallel microfluidic devices. (C) The cell suspension of iPS-derived hepatic progenitors cells were injected inside a microfluidic chip to form spheroids maturated into liver organoids. The maturation was followed by an increase on their average diameter during organoid culture. “Adapted with permission from Wang et al., 2020. Copyright 2020 American Chemical Society.” (D) Co-culture spheroids from human lung adenocarcinoma cells (A549) and endothelial cells (HUVECs) were transferred to a microfluidic device previously seeded with endothelial cells and lung fibroblasts embedded in a hydrogel. The tumor spheroids were capable of integration with the microvascular network and to drug response evaluated by Live and Dead assay. “Adapted with permission from Paek et al., 2019. Copyright 2019 American Chemical Society.” Both systems described the convergence of organoids and microfluidic, however, the increase of the complexity in (D) decreases its susceptibility to automation, large scale and reproducibility.