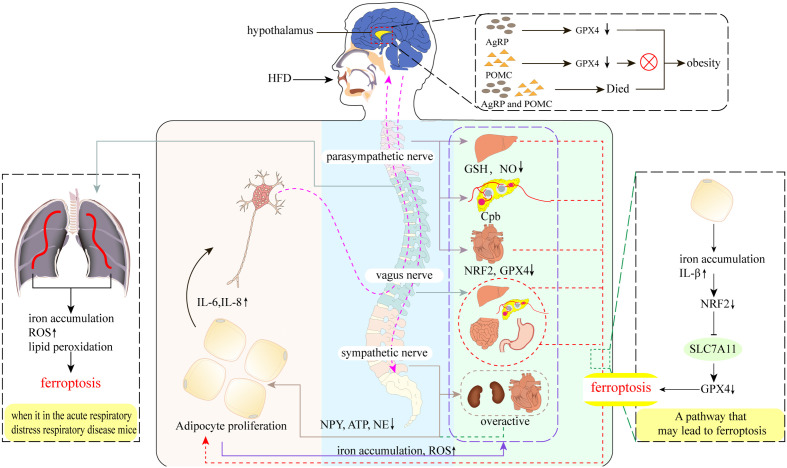
Figure 2.
Neural regulation of obesity and ferroptosis. A high-fat diet (HFD) leads to increased secretion of pro-inflammatory cytokines such as IL-6 and IL-8 in adipose tissue (AT), which stimulates AT sensory nerves and subsequent vagal-parasympathetic transmission to the nociceptors, which then transmit regulatory information directly to AT via sympathetic nerves, as well as by targeting tissues and organs elsewhere in the body to promote AT lipolysis and thermogenesis. However, the emergence of obesity disrupts these transmission pathways. Parasympathetic: obesity decreases hepatic GSH, NO levels, pancreatic cholinergic pathway block (Cpd), and cardiac NRF2 and GPX4 levels, which may cause ferroptosis in hepatic, pancreatic, and cardiac parasympathetic nerves, thus promoting AT amplification. Vagus nerve: part of the parasympathetic nerve. The presence of obesity may cause hepatic vagal ferroptosis, which further leads to possible ferroptosis of islet and intestinal and gastric vagal nerves. Sympathetic nerves: obesity leads to over-activation of sympathetic nerves, including cardiac and renal sympathetic nerves, and reduced levels of the sympathetic transmitters NPY, ATP, and NE, which also reduce their direct innervation of AT. Hypothalamus: AgRP neurons do not undergo ferroptosis after the knockdown of GPX4, do not undergo ferroptosis after knockdown, yet still resulted in the appearance of obesity, which was not observed in POMC neurons. Interestingly, AgRP and POMC neurons also led to obesity after inactivation.