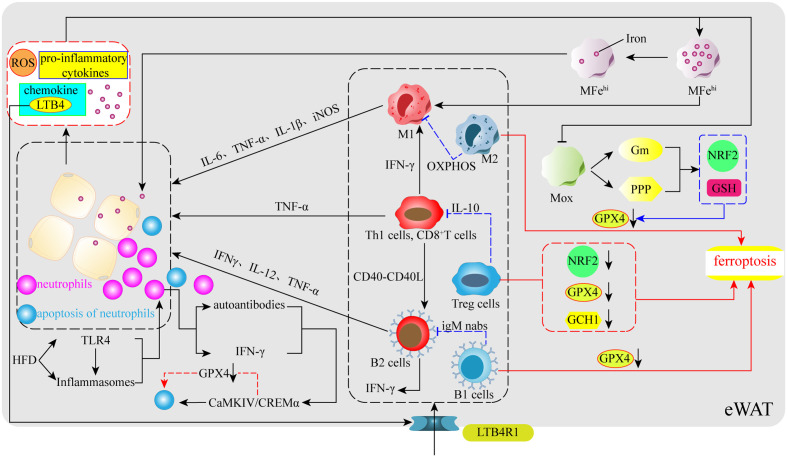
Figure 3.
Immunoregulation of obesity and ferroptosis. In the early stages of obesity, HFD stimulates TLR4, a sensor within VAT, and inflammatory vesicles to release certain signals to promote neutrophil entry into VAT, followed by the release of chemokines (LTB4) by neutrophils to further promote the aggregation of ATMs, T cells, and B cells in VAT. Simultaneously, their pro-inflammatory phenotype releases numerous pro-inflammatory cytokines, which increases the levels of ROS, iron, pro-inflammatory cytokines, adipocyte-secreted chemokines, and leads to increased inflammation due to ferroptosis in some types of anti-inflammatory immune cells. ATMs: obesity impairs the iron reserve capacity of MFehi macrophages and promotes their polarization toward the M1 phenotype. At the same time, a large number of pro-inflammatory cytokines also inhibit the upregulation of the NRF2 gene and GSH levels through glucose metabolism and the pentose phosphate pathway in Mox macrophages, which have antioxidant capacity, resulting in reduced GPX4 levels and ferroptosis in M2 macrophages. T cells: Obesity causes T cells to release IFN-γ and CD40-CD40L to promote M1 and B2 production. Tregs undergo ferroptosis due to reduced levels of NRF2, GPX4, and GCH1. B cells: obesity promotes the entry of B cells into VAT via the LTB4R1 receptor, whereas B1 cells may also undergo ferroptosis due to reduced GPX4. Neutrophils: neutrophils activate the CaMKIV/CREMα axis to induce apoptosis spontaneously, which may involve ferroptosis-related processes.