Abstract
Rationale & Objective
Patients with a high-risk Apolipoprotein L1 (APOL1) genotype are more likely to develop chronic kidney disease and kidney failure. It is unclear whether this increased risk is entirely mediated by the development of proteinuria.
Study Design
Retrospective observational study of the African American Study of Kidney Disease and Hypertension cohort and Chronic Renal Insufficiency Cohort.
Exposures & Predictors
Self-identified race (Black/non-Black) and presence of high-risk APOL1 genotype. The primary model was adjusted for age, sex, diabetes, estimated glomerular filtration rate, and urinary protein-creatinine ratio.
Outcomes
Time to kidney failure defined as time to dialysis or transplantation.
Analytical Approach
We used Cox proportional hazard models to study how proteinuria mediates the association between APOL1 and kidney failure. We modeled proteinuria at baseline and as a time-varying covariate.
Results
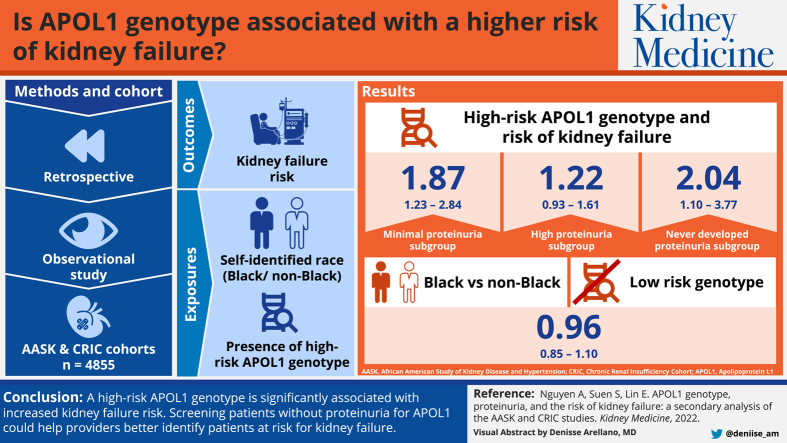
A high-risk APOL1 genotype was associated with a significantly higher risk of kidney failure, even for patients with minimal proteinuria (HR, 1.87; 95% CI, 1.23-2.84). The association was not significant among patients with high proteinuria (HR, 1.22; 95% CI, 0.93-1.61). When modeling proteinuria as a time-varying covariate, a high-risk APOL1 genotype was associated with higher kidney failure risk even among patients who never developed proteinuria (HR, 2.04; 95% CI, 1.10-3.77). Compared to non-Black patients, Black patients without the high-risk genotype did not have higher risk of kidney failure (HR, 0.96; 95% CI, 0.85-1.10).
Limitations
Two datasets were combined to increase statistical power. Limited generalizability beyond the study cohorts. Residual confounding common to observational studies.
Conclusions
A high-risk APOL1 genotype is significantly associated with increased kidney failure risk, especially among patients without baseline proteinuria. Although our results suggest that the risk is partially mediated through proteinuria, higher kidney failure risk was present even among patients who never developed proteinuria. Providers should consider screening for the high-risk APOL1 genotype, especially among Black patients without proteinuria in populations with chronic kidney disease.
Index Words: APOL1, CKD, proteinuria, kidney failure
Visual Abstract
Plain-Language Summary.
Some variants in the Apolipoprotein 1 (APOL1) gene are a major risk factor for developing kidney failure and are typically only found in Black individuals. However, it is unclear whether the increased risk from these “high-risk alleles” is due entirely to the development of proteinuria (protein in the urine). Data from 2 cohort studies of patients with chronic kidney disease were used to assess the association between APOL1 and kidney failure. We find that having high-risk alleles were associated with more kidney failure regardless of proteinuria. Moreover, Black patients without high-risk alleles were not more likely to develop kidney failure than non-Black patients. Screening patients without proteinuria for APOL1 could help providers better identify patients at risk for kidney failure.
Fifteen percent of US adults have chronic kidney disease (CKD), and Black Americans are disproportionately represented.1 Black Americans with CKD are 3 times more likely than non-Black Americans to progress to kidney failure.1
The high-risk Apolipoprotein L1 (APOL1) genotype (2 high-risk alleles), found in about 13%2 of Black Americans, is a major contributor to accelerated CKD progression.3, 4, 5, 6 The role that proteinuria plays in APOL1-mediated progression remains unclear. Some studies suggest that a high-risk APOL1 genotype predisposes patients to developing proteinuria; once present, proteinuria is the primary driver for kidney decline.6 Others suggest that even though a high-risk genotype increases patients’ risk for developing proteinuria, it might also contribute to kidney function decline independent of proteinuria.3, 4, 5 Understanding this mechanism is critical clinically because it could inform whether providers should regularly test patients for APOL1 high-risk alleles. For instance, even in the absence of proteinuria, providers may more closely monitor patients with a high-risk genotype for CKD progression and consider education and other preparation for kidney failure treatment. Prior studies investigating the relationship between APOL1 and proteinuria have been limited to short timeframes, do not account for longitudinal changes in proteinuria, and do not consider the persistence of severe levels of proteinuria.3, 4, 5, 6
In this study, we combined data from the African American Study of Kidney Disease and Hypertension (AASK) and the Chronic Renal Insufficiency Cohort (CRIC) cohort studies to investigate the long-term risk of kidney failure associated with the APOL1 gene. We modeled the time-varying nature of proteinuria to assess whether APOL1 is associated with kidney failure independent of proteinuria.
Methods
Overview
In this work, we developed 2 survival models to study the association between APOL1 and kidney failure risk. In Model 1, we studied the association between kidney failure risk with APOL1 status, age, sex, estimated glomerular filtration rate (eGFR), and patients’ baseline proteinuria level, measured as the urinary protein-creatinine ratio (UPCR). Because this first model only used baseline characteristics, it could not differentiate between patients who developed proteinuria after the baseline period and those who never developed proteinuria. To study whether APOL1 is associated with an increased risk of kidney failure even in patients who never developed proteinuria, we subsequently conducted an additional analysis (Model 2) that accounted for UPCR’s time-varying nature. Intuitively, these models compared patients who never developed proteinuria during the entire study period with those who did.
Data and Population
We used publicly available data from the AASK and CRIC cohort studies. Study protocols for each can be obtained from the National Institute of Diabetes and Digestive and Kidney Diseases Data Repository.7, 8, 9 This study was approved by University of Southern California institutional review board, approval number HS-18-00733. No informed consent was necessary for this study, as data were deidentified.
The AASK study included individuals who self-identified as Black, aged 18-70, and had CKD attributable to hypertension. The study had an initial trial phase where 1,094 participants were randomly assigned to different intensities of blood pressure control and different antihypertensive drug regimens.10 Of the 795 patients who did not develop kidney failure or die during the trial or transition period, 691 were followed for up to 5.4 years in a cohort study where all participants received the standard blood pressure regimen.10 Participants had environmental, genetic, physiologic, and socioeconomic factors collected annually.7 We studied the 691 participants in the cohort phase because they received standard of care.
The CRIC study is a longitudinal cohort of patients aged 21-74 with mild to moderate CKD (eGFR within 20-70 mL/min/1.73 m2).11,12 CRIC participants are followed every 6 months to ascertain interim medical history and to collect physiologic data.9 The maximum follow-up time was 14.5 years, and we used data collected through January 2020.
Our primary analysis combined both cohorts and adjusted for confounders common to both datasets. In a secondary analysis, we limited our analysis to the CRIC cohort, which has more complete information on sociodemographic and biomedical factors.
We required participants have complete information on APOL1 status, race (Black or non-Black), age, sex, UPCR, serum creatinine (used to calculate eGFR), and presence of diabetes. For the secondary CRIC-limited analysis and sensitivity analyses, we required patients have complete data on cystatin C and relevant sociodemographic and biomedical confounders.
Outcome
The outcome of interest was time to kidney failure defined as dialysis or transplant. We censored for loss to follow-up, death, and end of the study period.
Exposures
Our main exposures were self-identified race (Black vs non-Black) and the presence of a high-risk APOL1 genotype. We defined a high-risk genotype as having 2 high-risk alleles in the APOL1 gene (7.7% of our sample population). Because only Black patients have a high-risk APOL1 genotype, we examined 3 groups of patients: Black patients with a high-risk APOL1 genotype (having 2 high-risk alleles), Black patients without a high-risk APOL1 genotype (having 1 or no APOL1 high-risk alleles), and non-Black patients. We will refer to these groups as the Black-HR (Black high-risk), Black-LR (Black low-risk), and non-Black patients, respectively.
Main Prediction Variables
Because the Kidney Failure Risk Equation developed by Tangri et al13 is a widely used model for the risk stratification of patients with CKD, we based our initial model on the Kidney Failure Risk Equation 4-variable equation, which considers age, sex, eGFR, and albuminuria (measured through the urinary albumin-creatinine ratio [UACR]) as the main predictors for progression to kidney failure. We also included whether a patient was diagnosed with diabetes.
We estimated eGFR with the updated 2009 Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) creatinine equation refit without race.14,15 In the CRIC cohort, UACR is only available for the baseline visit, but the researchers collected longitudinal data on proteinuria through the UPCR. Prior studies have shown that UACR and UPCR are strongly correlated, so we used UPCR instead of UACR.16,17
Model 1 only incorporated patients’ baseline proteinuria, whereas Model 2 included proteinuria as a time-varying covariate. Both models modeled proteinuria as a categorical variable (“Minimal Proteinuria” or UPCR ≤ 0.220 mg/mg, “Moderate Proteinuria” or UPCR > 0.220 mg/mg to UPCR ≤ 1 mg/mg, and “High Proteinuria” or UPCR > 1 mg/mg). These cutoffs are similar to those used in prior studies.3 In sensitivity analysis, proteinuria was also modeled as a continuous variable (natural logarithm of UPCR).
Statistical Modeling
We conducted descriptive analyses comparing baseline characteristics among Black-HR, Black-LR, and non-Black patients. We used a Pearson χ2 test to compare categorical variables and a Kruskal-Wallis test to compare continuous variables. We plotted the probability of developing kidney failure over time using Kaplan-Meier survival curves, stratifying by level of proteinuria, using the log-rank test to test for statistical differences.
We subsequently used Cox proportional hazards models with robust standard errors to assess the association between APOL1 genotype, race, and risk of developing kidney failure after accounting for age (per 10 years), sex, eGFR (per 5 mL/min/1.73 m2), proteinuria, and diabetes.
In Model 1 (the “non–time-varying” model), we only included proteinuria at the baseline visit and an interaction term between the APOL1 high-risk genotype and proteinuria. This interaction term assesses whether the association between APOL1 and progression to kidney failure was heterogeneous by proteinuria level.
We then considered whether the future development of proteinuria might influence the association between the APOL1 high-risk genotype and kidney failure development. Model 2 (the “time-varying” categorical model) modeled proteinuria as a time-varying covariate.
We studied the association between APOL1 and the risk of developing kidney failure at different levels of proteinuria, using the delta method to obtain standard errors.
Sensitivity Analyses
We performed the following sensitivity analyses. First, we modeled proteinuria as a continuous variable (the natural logarithm of proteinuria). Second, the primary analysis was repeated with 3 alternative CKD-EPI equations.15 For some of these, only the CRIC data could be used because of the need for cystatin C data (Tables S1-S2, Item S1). Third, we used albuminuria instead of proteinuria. Albuminuria levels at the first visit were recorded and categorized as minimal (UACR ≤ 60 mg/g), moderate (60 mg/g < UACR ≤ 480 mg/g), and high (UACR > 480 mg/g). Albuminuria levels at follow-up visits were estimated using proteinuria via a previously published conversion equation by Sumida et al.16 Fourth, we assessed if other sociodemographic and biomedical factors confounded the results of the primary analysis. Most of these factors are only available in the CRIC data, so this analysis was performed exclusively on the CRIC cohort. In this analysis, we adjusted for sociodemographic and biomedical confounders in the CRIC cohort: biomedical confounders (ankle-brachial index, body mass index, ever visited a nephrologist, drinking in last 12 months, drug use, congestive heart failure, moderate depression, cause of kidney disease, family history of kidney disease or coronary artery disease, stroke, cardiovascular disease, atrial fibrillation, hypertension, and anemia) and sociodemographic confounders (Hispanic, health insurance, income, education, and marital status). We tested the sensitivity of our results when including: (1) biomedical confounders plus the base variables, (2) sociodemographic confounders plus the base variables, and (3) biomedical confounders, sociodemographic confounders, and base variables.
Results
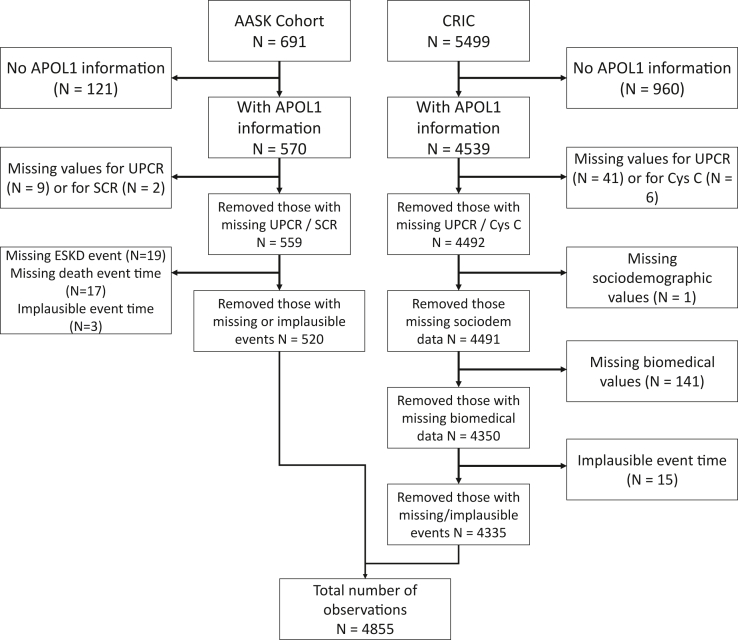
Of 6,190 individuals (691 from AASK and 5499 from CRIC), we included 4,855 in our study (520 from AASK and 4,335 from CRIC) (Fig 1). The maximum follow-up time for the included individuals was 5.4 years for the AASK participants and 14.5 years for the CRIC participants. As seen in Table 1, non-Black patients were older (mean age 60 versus 58 for Black-LR and 54 for Black-HR), more likely male (61% vs 53% for Black-LR and 49% for Black-HR), more likely to have diabetes (48% vs 40% for Black-LR and 31% for Black-HR), and more likely to have a higher mean eGFR (51 mL/min/1.73 m2 vs 40 mL/min/1.73 m2 for Black-LR and 39 mL/min/1.73 m2 for Black-HR). Non-Black patients and Black-LR patients were less likely to have proteinuria (41% and 40% respectively relative to 56% for Black-HR patients). Statistically significant differences were found for all variables among the 3 groups. Pairwise differences are presented with the full descriptive statistics in Table 1 and Table S1.
Figure 1.
Participant inclusion diagram. Abbreviations: AASK, African American Study of Kidney Disease and Hypertension; APOL1, Apolipoprotein L1; CRIC, Chronic Renal Insufficiency Cohort Study; Cys C, cystatin C; ESKD, end-stage kidney disease; SCR, serum to creatinine ratio; UPCR, urinary protein-creatinine ratio.
Table 1.
Descriptive Statistics for the Study Population (CRIC and AASK Studies)
| Non-Black (N = 2,911) | Black without APOL1 (Black-LR) (N = 1,570) |
Black with APOL1 (Black-HR) (N = 374) |
|
|---|---|---|---|
| Kidney failurea,b,c | 592 (20%) | 430 (27%) | 152 (41%) |
| Age (y)a,b,c | 59.6 (11.1) | 57.7 (9.9) | 54.0 (12.0) |
| Sexa,b | |||
| Male | 1,777 (61%) | 831 (52.9%) | 183 (48.9%) |
| Female | 1,134 (39%) | 739 (47.1%) | 191 (51.1%) |
| Baseline proteinuria levela,c | |||
| UPCR ≤ 0.220 mg/mg | 1,719 (59.1%) | 943 (60.1%) | 164 (43.9%) |
| 0.220 mg/mg < UPCR ≤ 1 mg/mg | 621 (21.3%) | 332 (21.1%) | 129 (34.5%) |
| UPCR > 1 mg/mg | 571 (19.6%) | 295 (18.8%) | 81 (21.7%) |
| Mean UPCR (mg/mg )a,b,c | 0.9 (2.3) | 0.8 (1.9) | 0.9 (1.9) |
| eGFR (mL/min/1.73 m2)a,b | 51.0 (16.2) | 39.6 (38.9) | 38.9 (15.1) |
| Diabetesa,b,c | |||
| Has diabetes | 1,389 (47.7%) | 621 (39.6%) | 114 (30.5%) |
Notes: Data are expressed as number (%) or mean (SD). Statistically significant differences are observed between all 3 groups for each characteristic.
Abbreviations: AASK, African American Study of Kidney Disease and Hypertension; APOL1, Apolipoprotein L1; eGFR, estimated glomerular filtration rate; Black-HR, Black with 2 high-risk APOL1 alleles; Black-LR, Black with one or no high-risk APOL1 alleles; CRIC, Chronic Renal Insufficiency Cohort Study; SD, standard deviation; UPCR, urinary protein-creatinine ratio.
Indicates significant difference between Non-Black and Black-LR
Indicates significant difference between Non-Black and Black-HR
Indicates significant difference between Black-LR and Black-HR
Unadjusted Analyses
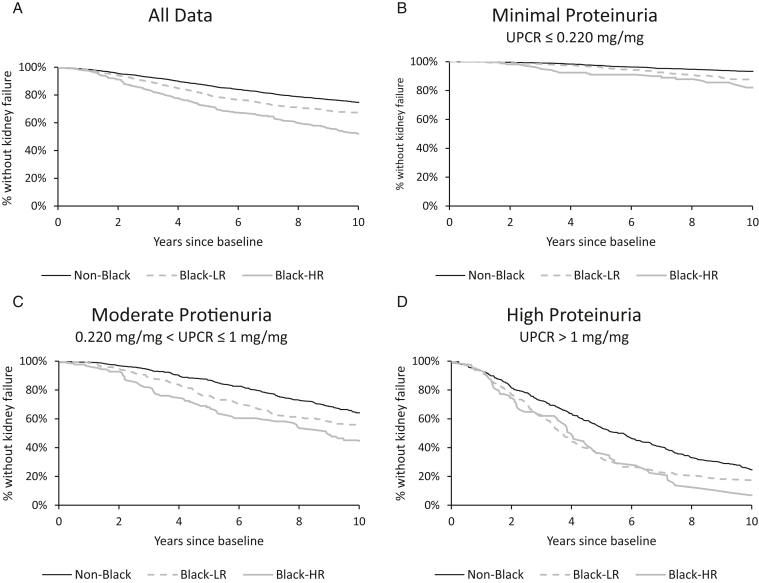
In unadjusted analysis, Black patients, and especially those with a high-risk APOL1 genotype, were significantly more likely to develop kidney failure than non-Black patients (Fig 2). When stratifying by proteinuria levels, we observed similar patterns. Black-HR patients progressed more quickly to kidney failure than Black-LR patients, and both groups progressed more quickly than non-Black patients. For patients with minimal proteinuria, the likelihood of kidney failure for non-Blacks versus Black-LRs was similar in the first 5 years but diverged by 10 years of follow-up. For patients with high proteinuria levels, the likelihood of kidney failure was similar for the first 7 years but diverged by 10 years of follow-up.
Figure 2.
(A and B) Patients with the high-risk APOL1 genotype have higher risk of kidney failure even when stratifying by proteinuria. Survival curves constructed using the Kaplan-Meier estimator. Log-rank tests indicated that at the 2 higher UPCR levels, plots (C) and (D), there is no statistically significant difference between Black with APOL1 and Black without APOL1 subgroups. All other pairwise comparisons showed statistically significant differences (P < 0.01). Abbreviations: APOL1, Apolipoprotein L1; Black-HR, Black with 2 high-risk APOL1 alleles; Black-LR, Black with 1 or no high-risk APOL1 alleles; eGFR, estimated glomerular filtration rate; UPCR, urinary protein-creatinine ratio.
Primary Adjusted Analyses
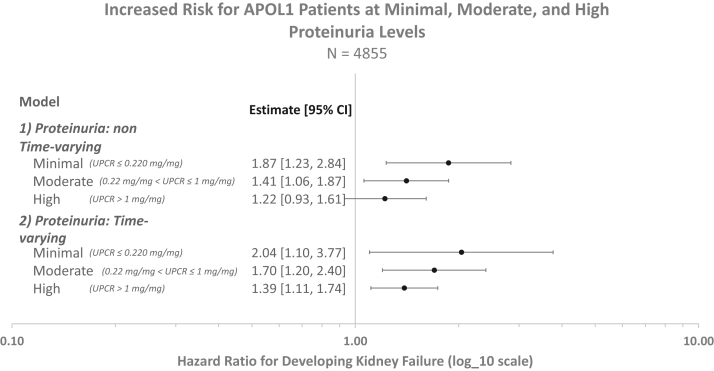
After adjusting for age, sex, eGFR, and presence of diabetes, we found that having a high-risk APOL1 genotype was associated with a significantly higher risk of developing kidney failure at all baseline levels of proteinuria (Model 1 in Table 2, Fig 3). Figure 3 illustrates how the high-risk APOL1 genotype is associated with increased kidney failure risk across different proteinuria strata. The association is blunted at more severe proteinuria levels, although the interaction term was not significant at the 5% level (Table 2). The APOL1 high-risk genotype was associated with higher kidney failure risk among patients with minimal (hazard ratio [HR], 1.87; 95% confidence interval [CI], 1.23-2.84) and moderate (HR, 1.41; 95% CI, 1.06-1.87) proteinuria at baseline, but not among patients with high (HR, 1.22; 95% CI, 0.93-1.61) levels of proteinuria (Table S2).
Table 2.
Higher Risk of Kidney Failure Associated with APOL1 and Proteinuria
| Variables | Model 1 Proteinuria: non–time-varying |
Model 2 Proteinuria: time-varying |
||
|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | |
| Race | ||||
| Non-Black | Ref | Ref | ||
| Black | 0.963 | (0.847-1.095) | 0.906 | (0.797-1.029) |
| APOL1 genotype | ||||
| Low-risk (0-1 allele mutation) | Ref | Ref | ||
| High-risk (2 allele mutation) | 1.875 | (1.237-2.842)a | 2.040 | (1.103-3.772)b |
| Proteinuria | ||||
| Minimal (UPCR ≤ 0.220 mg/mg) | Ref | Ref | ||
| Moderate (0.22 mg/mg < UPCR ≤ 1 mg/mg) | 3.458 | (2.871-4.165)a | 4.222 | (3.228-5.523)a |
| High (UPCR > 1 mg/mg) | 9.747 | (8.159-11.644)a | 15.830 | (12.381-20.241)a |
| APOL1 × proteinuriac | ||||
| APOL1 high-risk × moderate proteinuria | 0.733 | (0.448-1.199) | 0.831 | (0.414-1.668) |
| APOL1 high-risk × severe proteinuria | 0.636 | (0.391-1.035) | 0.680 | (0.357-1.297) |
| Age (per 10 y) | 0.824 | (0.781-0.868)a | 0.789 | (0.748-0.831)a |
| Sex | ||||
| Female | Ref | Ref | ||
| Male | 1.343 | (1.194-1.511)a | 1.222 | (1.086-1.376)a |
| eGFR (per change in 5 mL/min/1.73 m2)d | 0.688 | (0.670-0.707)a | 0.695 | (0.675-0.716)a |
| Presence of diabetes | ||||
| No | Ref | Ref | ||
| Yes | 1.628 | (1.439-1.842)a | 1.636 | (1.447-1.851)a |
Notes: Two Cox models were developed. Proteinuria was modeled in 2 different ways: (1) proteinuria level at baseline, and (2) proteinuria modeled as a time-varying covariate. These models use both CRIC and AASK data (N = 4,855). HRs estimated using a Cox proportional hazards model, adjusting for the above covariates. Higher risk of kidney failure is associated with proteinuria and APOL1. Age is measured in years, and eGFR is measured in mL/min/1.73 m2. Age HR is per 10 years and eGFR HR is per 5 mL/min/1.73 m2.
Abbreviations: AASK, African American Study of Kidney Disease and Hypertension; APOL1, Apolipoprotein L1; CI, confidence interval; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; CRIC, Chronic Renal Insufficiency Cohort Study; eGFR, estimated glomerular filtration rate; HR, hazard ratio; Ref, reference; UPCR, urinary protein-creatinine ratio.
Significant at 0.01
Significant at 0.05
Interaction between the presence of a high-risk APOL1 genotype and proteinuria level
Estimated using CKD-EPI equation without race fit
Figure 3.
Increased risk of kidney failure for patients with the high-risk APOL1 alleles even for patients with minimal proteinuria. Estimates derived from a Cox proportional hazards model, adjusting for age, sex, diabetes, estimated glomerular filtration rate, and UPCR. Proteinuria levels are defined as: Minimal (UPCR ≤ 0.22 mg/mg), Moderate (0.22 mg/mg < UPCR ≤ 1 mg/mg), and High (UPCR >1 mg/mg). Both models show that there is an increased risk for kidney failure at all proteinuria levels if the patient has the high-risk APOL1 allele. Abbreviations: APOL1, Apolipoprotein L1; UPCR, urinary protein-creatinine ratio.
Model 2, which allowed proteinuria to vary over time, showed similar results: a high-risk APOL1 genotype was associated with a higher kidney failure risk, again blunted at higher levels of proteinuria (Fig 2). Interaction terms for APOL1 and proteinuria were not significant (Table 2). In contrast to Model 1, patients with the high-risk APOL1 genotype and who developed high proteinuria at any point during the study period had a higher risk of kidney failure (Model 2: HR, 1.39; 95% CI, 1.11-1.74). We additionally found that APOL1 was associated with kidney failure risk even for patients who never developed proteinuria during the study period (Model 2: HR, 2.04; 95% CI, 1.10-3.77; Table S2).
Sensitivity Analyses
In sensitivity analyses, we did not observe material changes to our results regarding APOL1 and proteinuria when modeling proteinuria as a continuous variable or when using any of the other eGFR estimation equations (Table S2). When using UACR instead of UPCR, our results were consistent. We explored the impact of including biomedical and sociodemographic factors (see Table S1 for descriptive statistics). In all cases, including these additional confounders does not materially change our adjusted results. HRs for the stratified proteinuria groups can be found in Table S2. Relevant variable HRs can be found in Table S2.
Discussion
We found that having a high-risk APOL1 genotype was associated with significantly increased kidney failure risk, especially among patients with lower levels of proteinuria. The association between the high-risk APOL1 genotype and kidney failure was blunted at higher levels of proteinuria, though it remained significant among patients who eventually developed severe proteinuria during the study period. Notably, the high-risk genotype was associated with higher kidney failure risk even among patients who never developed proteinuria during the study period.
A leading hypothesis for how the high-risk APOL1 genotype causes CKD progression is through the development of proteinuria. Prior studies have suggested that among patients without proteinuria, the presence of a high-risk APOL1 genotype significantly increases patients’ future risk for developing proteinuria, a finding that is consistent with ours.5 Previous research into the mechanism of how APOL1 accelerates CKD progression have focused a large part on the development of proteinuria. However, we find in this study that faster CKD progression rates were associated with the high-risk APOL1 genotype even when patients do not have proteinuria. Our findings are consistent with the translational literature suggesting nonglomerular mechanisms for APOL1-mediated kidney damage, including endoplasmic reticular stress, mitochondrial dysfunction, and cytotoxicity resulting in apoptosis and pyroptosis, among others.18, 19, 20, 21 Future studies should investigate additional mechanisms independent of the development of proteinuria. Although kidney damage from the high-risk APOL1 genotype is mediated in part by the development of proteinuria, kidney damage may occur through a mechanism independent of proteinuria.
Our study extends current knowledge about the APOL1 gene in several ways. First, unlike previous studies that focused on short term outcomes (2 and 5 years),13 ours considers a longer follow-up period, up to 14 years after the initial visit. Additionally, previous studies considered proteinuria as a static characteristic measured once during a baseline visit.22, 23, 24, 25, 26, 27 However, proteinuria changes occur over time, and accounting for longitudinal changes in proteinuria may be important.28,29 Our time-varying models suggest that the high-risk APOL1 genotype is an important risk factor for developing kidney failure even among patients who never developed proteinuria. These findings were robust to multiple statistical formulations.
The literature remains unclear about whether screening patients for APOL1 high-risk alleles conveys a clinical benefit and acknowledge that the paucity of therapeutic options for APOL1-mediated damage may limit the benefits of more APOL1 testing.30 However, testing may help providers and patients understand why a patient might have progressive, nonproteinuric kidney disease. Moreover, in light of our results, providers may want to reconsider the utility of using high-risk APOL1 genotype, even in the absence of proteinuria, as a factor when risk stratifying patients. This could be valuable in limited resource or safety-net settings where optimizing the allocation of intensive and expensive but cost-effective therapies could improve overall population management of patients with kidney disease. Prior studies have shown that cost effectiveness of management of CKD varies drastically across risk profiles and that risk stratification has the potential to improve the cost-effective care by allowing providers to target more intensive therapies to patients at the highest risk for kidney failure.31 The presence of a high-risk genotype could raise a provider’s index of suspicion for future CKD progression and could prompt more frequent testing for evidence of CKD progression. Many clinics have already included the Kidney Failure Risk Equation as a way to stratify patients,32, 33, 34, 35 and APOL1 may serve as a valuable additional factor for risk stratification. The utility of APOL1 may be even higher for younger patients who have not developed proteinuria but have a longer life expectancy, increasing the likelihood of developing kidney failure. This approach should be confirmed in future studies.
Screening for APOL1 might also change the management of kidney disease considering the abundance of evidence demonstrating the efficacy of sodium/glucose cotransporter 2 inhibitors. Early trials demonstrated improved kidney outcomes, even among subgroups of patients with higher eGFRs and without proteinuria.36,37 Recent studies have demonstrated the benefit of sodium/glucose cotransporter 2 inhibitors in both diabetic and nondiabetic CKD, but most of these trials have required that patients have macroalbuminuria.38 Although a randomized trial has not yet definitively demonstrated benefit in patients with nonproteinuric CKD, sodium/glucose cotransporter 2 inhibitors might confer benefits for patients without proteinuria but with the high-risk APOL1 genotype. A positive APOL1 test may prompt a provider to control blood pressure more aggressively or use an sodium/glucose cotransporter 2 inhibitor in an otherwise healthy patient without proteinuria.
Our study might also have important implications in the recruitment of patients for trials testing novel therapeutics to treat APOL1-mediated focal segmental glomerulosclerosis. Many of these studies have proteinuria in their eligibility criteria. Although patients with proteinuria are of the highest risk for developing kidney failure, these patients have already suffered substantial glomerular damage. Trials could consider testing the efficacy of therapeutics among patients with a high-risk APOL1 genotype but who have not yet developed proteinuria. It is important to test whether moving upstream is more efficacious in ameliorating the future risk of kidney failure.
Limitations of our study stem from data availability and the use of multiple cohort studies for our study. A large portion of the CRIC cohort lacked APOL1 data and were excluded from our study. We also used proteinuria as a proxy for albuminuria because of the lack of longitudinal data on albuminuria in the CRIC dataset. Although our study is unique in modeling proteinuria as a time-varying covariate, we could only update proteinuria levels annually given the follow-up schedules of both cohorts. Additionally, our results may not be fully generalizable to the entire US CKD population given the selective inclusion criteria for the AASK and CRIC cohorts. For instance, some Black patients with a high-risk genotype might not have CKD due to APOL1 but rather other risk factors (eg, diabetes). In a similar vein, both cohorts omitted patients with a high-risk genotype but no evidence of CKD (ie, an absence of proteinuria and normal eGFR). As with all observational studies, our findings could be biased from residual confounding. However, given the size of the point estimates, the robustness of our results to multiple model specifications, and the relatively small impact of including confounders on our results, the bias may not be sufficient to invalidate our results. We also excluded the AASK population in our exploratory secondary analyses because many confounders were limited to the CRIC dataset. Finally, although we used 2 rich cohorts with a long follow-up period, our small sample size may have led to imprecise estimates.
In summary, we find that APOL1 is significantly associated with the long-term risk of developing kidney failure, even for patients without underlying proteinuria in populations with CKD. Although proteinuria may be one mechanism by which APOL1 leads to kidney failure, our findings suggest the importance of confirming whether APOL1-mediated kidney damage could stem from alternate mechanisms. Providers should consider the value using APOL1 high-risk alleles as a risk-stratifying characteristic.
Article Information
Authors’ Full Names and Academic Degrees
Anthony Nguyen, PhD, Sze-chuan Suen, PhD, and Eugene Lin, MD
Authors’ Contributions
Study conception: EL; methodology design: AN, SS, and EL; statistical analysis: AN; supervision/mentorship: SS and EL; data interpretation: AN, SS, and EL. Funding acquisition and data resources management: EL and SS. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Support
This research was supported in part by the James H. Zumberge Faculty Research and Innovation Fund at the University of Southern California. This work was also supported in part by the National Institutes of Health (NIH) through the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK): EL receives support from NIDDK K08DK118213. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health nor any other funders. None of the funders had any role in study design, data collection or analysis, interpretation of data, writing the report, or the decision to submit these data for publication.
Financial Disclosure
The authors declare that they have no relevant financial interests.
Acknowledgements
Data from this study came from a publicly available repository supplied by the National Institute of Diabetes and Digestive and Kidney Diseases.
Peer Review
Received April 27, 2022 as a submission to the expedited consideration track with 2 external peer reviews. Direct editorial input from the Statistical Editor and the Editor-in-Chief. Accepted in revised form August 28, 2022.
Footnotes
Complete author and article information provided before references.
Item S1: Calculations for eGFR and UPCR to UACR
Table S1: Descriptive Statistics for AASK and CRIC Data Individually
Table S2: Relevant Hazard Ratios from Sensitivity Analysis
Supplementary Material
Item S1, Table S1-S2.
References
- 1.Centers for Disease Control and Prevention . US Department of Health and Human Services; Atlanta, GA: 2019. Chronic Kidney Disease in the United States, 2019.https://www.cdc.gov/kidneydisease/pdf/2019_National-Chronic-Kidney-Disease-Fact-Sheet.pdf [Google Scholar]
- 2.Dummer P.D., Limou S., Rosenberg A.Z., et al. APOL1 kidney disease risk variants: an evolving landscape. Semin Nephrol. 2015;35(3):222–236. doi: 10.1016/j.semnephrol.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parsa A., Kao W.H.L., Xie D., et al. APOL1 risk variants, race, and progression of chronic kidney disease. N Engl J Med. 2013;369(23):2183–2196. doi: 10.1056/NEJMoa1310345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Foster M.C., Coresh J., Fornage M., et al. APOL1 variants associate with increased risk of CKD among African Americans. J Am Soc Nephrol. 2013;24(9):1484–1491. doi: 10.1681/ASN.2013010113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peralta C.A., Bibbins-Domingo K., Vittinghoff E., et al. APOL1 genotype and race differences in incident albuminuria and renal function decline. J Am Soc Nephrol. 2016;27(3):887–893. doi: 10.1681/ASN.2015020124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen T.K., Tin A., Peralta C.A., et al. APOL1 risk variants, incident proteinuria, and subsequent eGFR decline in blacks with hypertension-attributed CKD. Clin J Am Soc Nephrol. 2017;12(11):1771–1777. doi: 10.2215/CJN.01180117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Institute of Diabetes and Digestive and Kidney Diseases African American Study of Kidney Disease and Hypertension Cohort Study (AASK Cohort) https://repository.niddk.nih.gov/studies/aask-cohort/
- 8.National Institute of Diabetes and Digestive and Kidney Diseases African American Study of Kidney Disease and Hypertension Study (Clinical Trial) (AASK Trial) https://repository.niddk.nih.gov/studies/aask-trial/
- 9.National Institute of Diabetes and Digestive and Kidney Diseases Chronic Renal Insufficiency Cohort Study (CRIC) https://repository.niddk.nih.gov/studies/cric/
- 10.Sika M., Lewis J., Douglas J., et al. Baseline characteristics of participants in the African American study of kidney disease and hypertension (AASK) clinical trial and cohort study. Am J Kidney Dis. 2007;50(1):78–89. doi: 10.1053/j.ajkd.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 11.Chronic Renal Insufficiency Cohort Study About CRIC. http://www.cristudy.org/Chronic-Kidney-Disease/Chronic-Renal-Insufficiency-Cohort-Study/about
- 12.Lash J.P., Go A.S., Appel L.J., et al. Chronic Renal Insufficiency Cohort (CRIC) study: baseline characteristics and associations with kidney function. Clin J Am Soc Nephrol. 2009;4(8):1302–1311. doi: 10.2215/CJN.00070109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tangri N., Grams M.E., Levey A.S., et al. Multinational assessment of accuracy of equations for predicting risk of kidney failure: a meta-analysis. JAMA. 2016;315(2):164–174. doi: 10.1001/jama.2015.18202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levey A.S., Stevens L.A. Estimating GFR using the CKD Epidemiology Collaboration (CKD-EPI) creatinine equation: more accurate GFR estimates, lower CKD prevalence estimates, and better risk predictions. Am J Kidney Dis. 2010;55(4):622–627. doi: 10.1053/j.ajkd.2010.02.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Inker L.A., Eneanya N.D., Coresh J., et al. New creatinine- and cystatin C–based equations to estimate GFR without race. N Engl J Med. 2021;385(19):1737–1749. doi: 10.1056/NEJMoa2102953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sumida K., Nadkarni G.N., Grams M.E., et al. Conversion of urine protein-creatinine ratio or urine dipstick protein to urine albumin-creatinine ratio for use in chronic kidney disease screening and prognosis: an individual participant-based meta-analysis. Ann Intern Med. 2020;173(6):426–435. doi: 10.7326/M20-0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Naranjo F.S., Sang Y., Ballew S.H., et al. Estimating kidney failure risk using electronic medical records. Kidney360. 2021;2(3):415–424. doi: 10.34067/KID.0005592020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wen H., Kumar V., Lan X., et al. APOL1 risk variants cause podocytes injury through enhancing endoplasmic reticulum stress. Biosci Rep. 2018;38(4) doi: 10.1042/BSR20171713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gerstner L., Chen M., Kampf L.L., et al. Inhibition of endoplasmic reticulum stress signaling rescues cytotoxicity of human apolipoprotein-L1 risk variants in Drosophila. Kidney Int. 2022;101(6):1216–1231. doi: 10.1016/j.kint.2021.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar V., Singhal P.C. APOL1 and kidney cell function. Am J Physiol Renal Physiol. 2019;317(2):F463–F477. doi: 10.1152/ajprenal.00233.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma L., Chou J.W., Snipes J.A., et al. APOL1 renal-risk variants induce mitochondrial dysfunction. J Am Soc Nephrol. 2017;28(4):1093–1105. doi: 10.1681/ASN.2016050567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jardine M.J., Hata J., Woodward M., et al. Prediction of kidney-related outcomes in patients with type 2 diabetes. Am J Kidney Dis. 2012;60(5):770–778. doi: 10.1053/j.ajkd.2012.04.025. [DOI] [PubMed] [Google Scholar]
- 23.O’Seaghdha C.M., Yang Q., Wu H., Hwang S.J., Fox C.S. Performance of a genetic risk score for CKD stage 3 in the general population. Am J Kidney Dis. 2012;59(1):19–24. doi: 10.1053/j.ajkd.2011.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ando M., Yanagisawa N., Ajisawa A., Tsuchiya K., Nitta K. A simple model for predicting incidence of chronic kidney disease in HIV-infected patients. Clin Exp Nephrol. 2011;15(2):242–247. doi: 10.1007/s10157-010-0393-x. [DOI] [PubMed] [Google Scholar]
- 25.Halbesma N., Jansen D.F., Heymans M.W., et al. Development and validation of a general population renal risk score. Clin J Am Soc Nephrol. 2011;6(7):1731–1738. doi: 10.2215/CJN.08590910. [DOI] [PubMed] [Google Scholar]
- 26.Chien K.L., Lin H.J., Lee B.C., Hsu H.C., Lee Y.T., Chen M.F. A prediction model for the risk of incident chronic kidney disease. Am J Med. 2010;123(9):836–846.e2. doi: 10.1016/j.amjmed.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 27.Cerqueira D.C., Soares C.M., Silva V.R., et al. A predictive model of progression of CKD to ESRD in a predialysis pediatric interdisciplinary program. Clin J Am Soc Nephrol. 2014;9(4):728–735. doi: 10.2215/CJN.06630613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Usui T., Kanda E., Iseki C., Iseki K., Kashihara N., Nangaku M. Observation period for changes in proteinuria and risk prediction of end-stage renal disease in general population. Nephrology (Carlton) 2018;23(9):821–829. doi: 10.1111/nep.13093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lea J., Greene T., Hebert L., et al. The relationship between magnitude of proteinuria reduction and risk of end-stage renal disease: results of the African American study of kidney disease and hypertension. Arch Intern Med. 2005;165(8):947–953. doi: 10.1001/archinte.165.8.947. [DOI] [PubMed] [Google Scholar]
- 30.Groopman E.E., Rasouly H.M., Gharavi A.G. Genomic medicine for kidney disease. Nat Rev Nephrol. 2018;14(2):83–104. doi: 10.1038/nrneph.2017.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin E., Chertow G.M., Yan B., Malcolm E., Goldhaber-Fiebert J.D. Cost-effectiveness of multidisciplinary care in mild to moderate chronic kidney disease in the United States: A modeling study. PLOS Med. 2018;15(3) doi: 10.1371/journal.pmed.1002532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hemmelgarn B.R., Smekal M.D., Weaver R.G., et al. Implementation and evaluation of a risk-based approach to guide chronic kidney disease care: protocol for a multiphase mixed-methods study. Can J Kidney Health Dis. 2018;5 doi: 10.1177/2054358117753618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harasemiw O., Drummond N., Singer A., et al. Integrating risk-based care for patients with chronic kidney disease in the community: study protocol for a cluster randomized trial. Can J Kidney Health Dis. 2019;6 doi: 10.1177/2054358119841611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smekal M.D., Tam-Tham H., Finlay J., et al. Perceived benefits and challenges of a risk-based approach to multidisciplinary chronic kidney disease care: a qualitative descriptive study. Can J Kidney Health Dis. 2018;5 doi: 10.1177/2054358118763809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smekal M.D., Tam-Tham H., Finlay J., et al. Patient and provider experience and perspectives of a risk-based approach to multidisciplinary chronic kidney disease care: a mixed methods study. BMC Nephrol. 2019;20(1):110. doi: 10.1186/s12882-019-1269-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wiviott S.D., Raz I., Bonaca M.P., et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347–357. doi: 10.1056/NEJMoa1812389. [DOI] [PubMed] [Google Scholar]
- 37.Zinman B., Wanner C., Lachin J.M., et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 38.Heerspink H.J.L., Stefánsson B.V., Correa-Rotter R., et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383(15):1436–1446. doi: 10.1056/NEJMoa2024816. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Item S1, Table S1-S2.