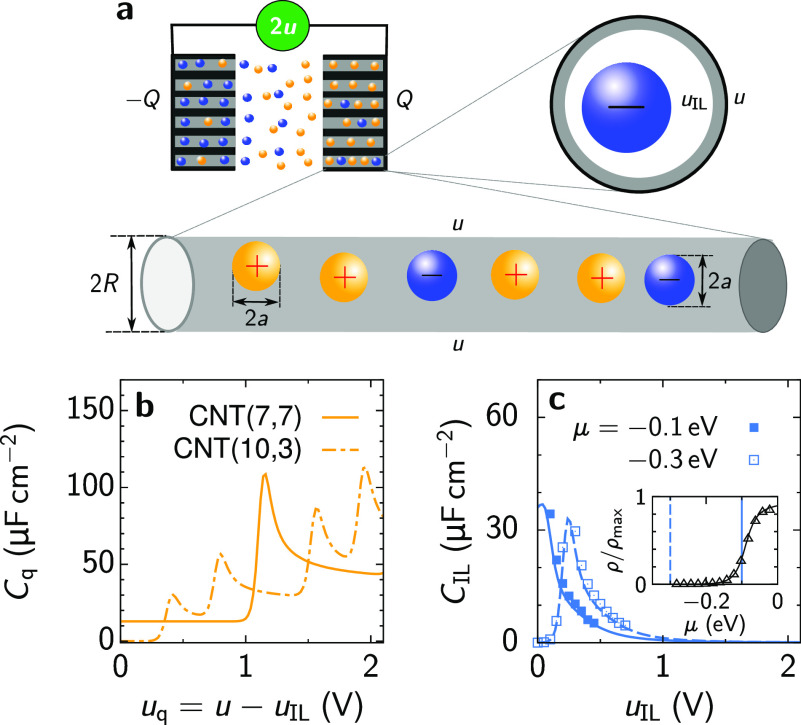
Figure 1.
Ionic liquids in carbon nanotubes (CNTs) and examples of quantum and electrical double-layer capacitances. (a) Schematic of a supercapacitor with two nanoporous electrodes and ions of the same radius a. A potential u is applied to the outer surfaces of CNTs, as measured with respect to the bulk electrolyte; uIL is the potential at the inner CNT surface. The bottom and top right cartoons show the side and top views, respectively, of a single CNT filled with ions. The radius of a CNT is R as measured to the center of the carbon atoms. (b) Examples of voltage-dependent quantum capacitance Cq calculated using the Mintmire–White formula for the CNT density of states19 (section S1). CNT(7,7) is metallic, and CNT(10,3) is semiconducting; the latter has zero capacitance inside the band gap (around zero voltage). The CNT radii are ≈0.47 and 0.46 nm, respectively. T = 300 K. (c) Examples of voltage-dependent electrical double-layer capacitance CIL calculated using the analytical solution of ref (26) (lines) and 3D Monte Carlo simulations (symbols). The ion radius a = 0.25 nm, the tube radius R = 0.47 nm, the in-pore dielectric constant ε = 5, and T = 300 K. The inset shows the 1D total ion density at zero voltage as a function of the ion chemical potential μ; ρmax = (2a)−1 is the maximum 1D density. The thin vertical lines show the μ values of −0.1 and −0.3 eV used in the main plot; these values correspond to a moderately ionophilic and ionophobic pore, characterized by high and vanishing in-pore ion density, respectively, as the inset demonstrates (see also Figure S1).