FIGURE.
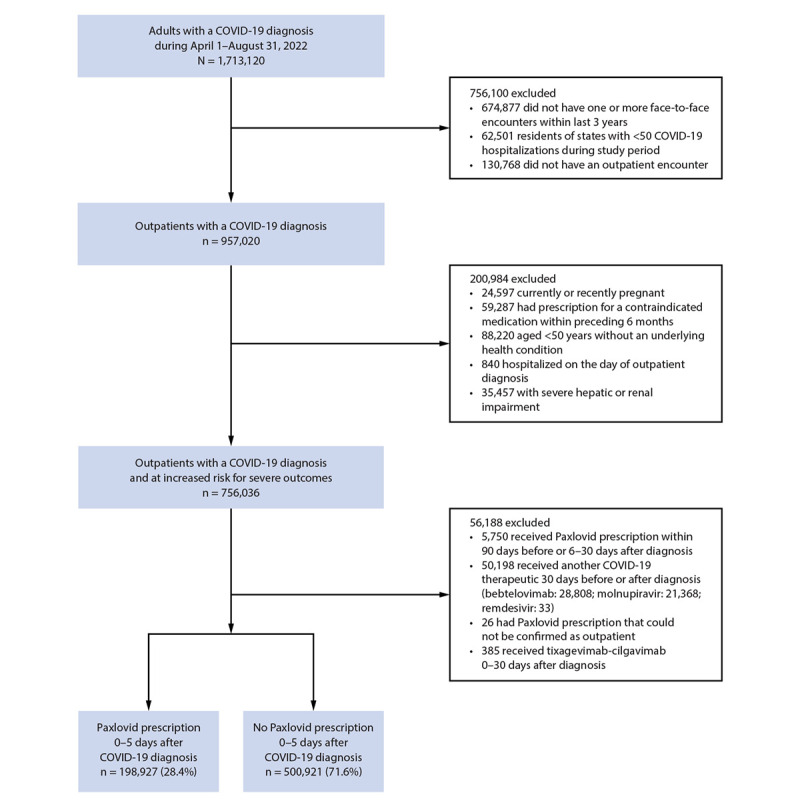
Identification of patients with COVID-19* who were eligible for treatment with Paxlovid (nirmatrelvir-ritonavir) — Cosmos,† United States, April–September 2022
Abbreviation: NAAT = nucleic acid amplification test.
* Patients were classified as having COVID-19 based on a diagnosis code for COVID-19 or based on a positive SARS-CoV-2 antigen or nucleic acid amplification test. Among 1,713,120 adults aged ≥18 years who met this definition during April 1–August 1, 2022, 930,847 had a diagnosis code only, 159,878 had a positive NAAT result only, 12,874 had a positive antigen test result only, and 609,521 had both a diagnosis code and positive test result (NAAT or antigen test). Exclusions summarized at each level of the flow chart are not mutually exclusive.
† Cosmos is an electronic health record dataset that includes information from >160 million persons in U.S. health systems covered by Epic. https://cosmos.epic.com
