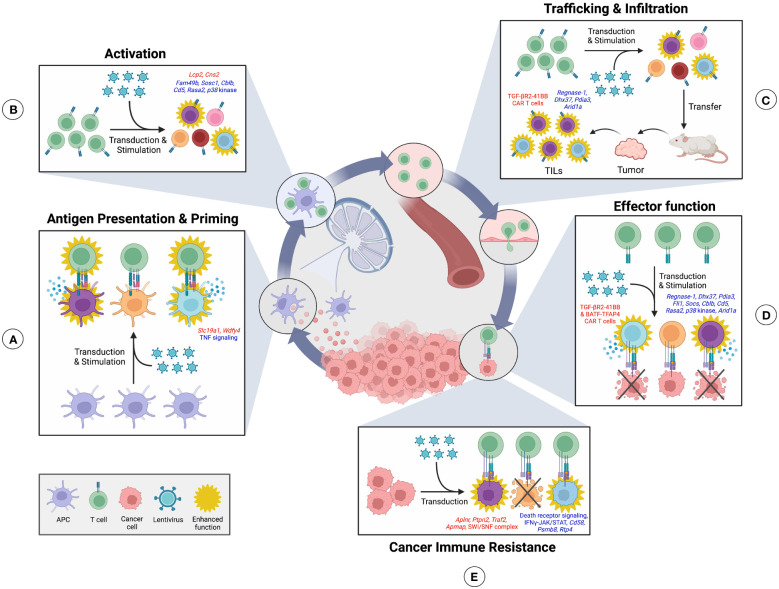
Figure 1.
CRISPR screens can identify regulators of the Cancer-immunity cycle. The cancer-immunity cycle is a framework which describes the sequential generation of antitumor immune responses. High-throughput CRISPR screening can be used to screen cells in each step of this cycle to discover regulatory genes and their corresponding phenotypic effect. (A) In this cycle, tumor antigens are first released by cancer cells and sampled by antigen-presenting cells (APCs), such as dendritic cells, that may release cytokines in response to stimulation. APCs can then process and present captured antigens using major histocompatibility complex proteins on their surface. Trafficking of APCs to nearby lymph nodes allows for presentation of cancer antigens to naïve T cells for subsequent T cell activation. Screens on APCs can uncover genes regulating APC stimulation in response to tumor antigens and antigen presentation efficiency to T cells. (B) T cells that receive antigen stimulation become primed and activated towards a given tumor antigen. T cell screens can identify genes that mediate activation efficiency. (C) Primed T cells, such as cytotoxic T lymphocytes, can then egress from the lymph node, migrate through the blood, and infiltrate the tumor as tumor infiltrating lymphocytes (TILs). In vivo T cell screens can discover genes that promote TIL trafficking and infiltration. (D) Within the tumor, T cells are finally able to recognize their cognate cancer-specific antigen and induce tumor cell killing. T cell screens can identify genes that enhance tumor-killing activity. (E) Meanwhile, screening of tumor cells can uncover genes that mediate resistance to T cell killing. Positive regulators discovered at each step using CRISPR screens are shown in red, while negative regulators are shown in blue.