Figure 1.
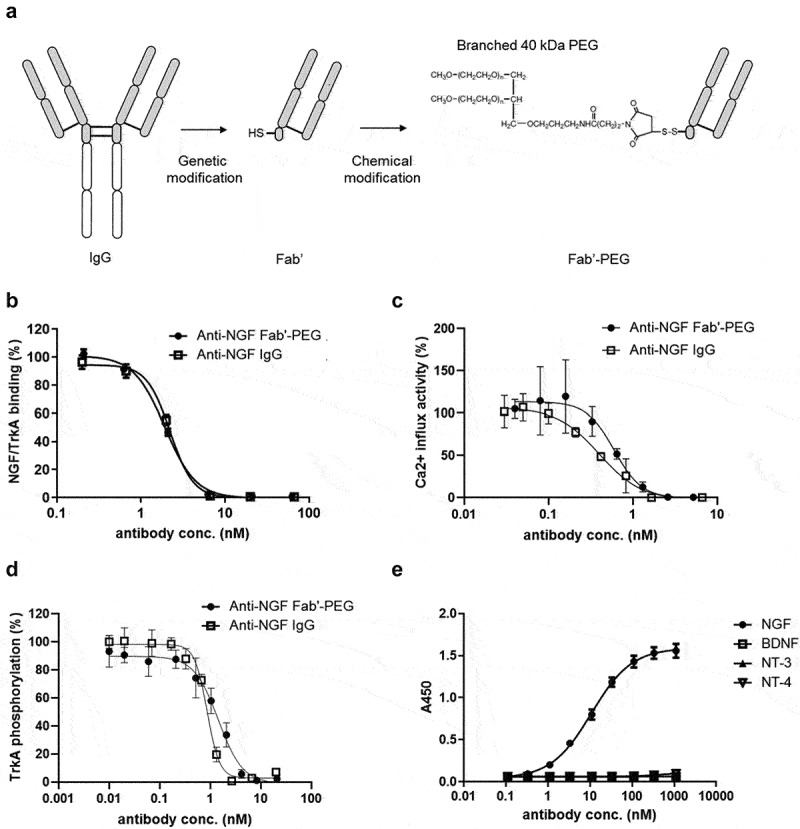
Generation and characterization of a novel anti-NGF Fab’-PEG. (a) Scheme showing how the Fab’-PEG was generated. (b) Inhibitory activity of NGF/TrkA binding in competitive ELISA. (c) The inhibitory activity of NGF induced a calcium influx in TrkA-expressing HEK293 cells. (d) The inhibitory activity of NGF induced TrkA phosphorylation in TrkA-expressing HEK293 cells. Values indicate percent activity, with the effect of NGF alone (without antibody) representing 100%, and the basal activity without NGF or maximum concentration of anti-NGF Fab’-PEG defined as 0%. (e) Binding activity of the anti-NGF Fab’-PEG to human NGF, BDNF, NT-3 and NT-4. Values indicate absorbance at 450 nm. Data are presented as mean ± SD.
