Figure 3. The binding mode of OTS964 to CDK11.
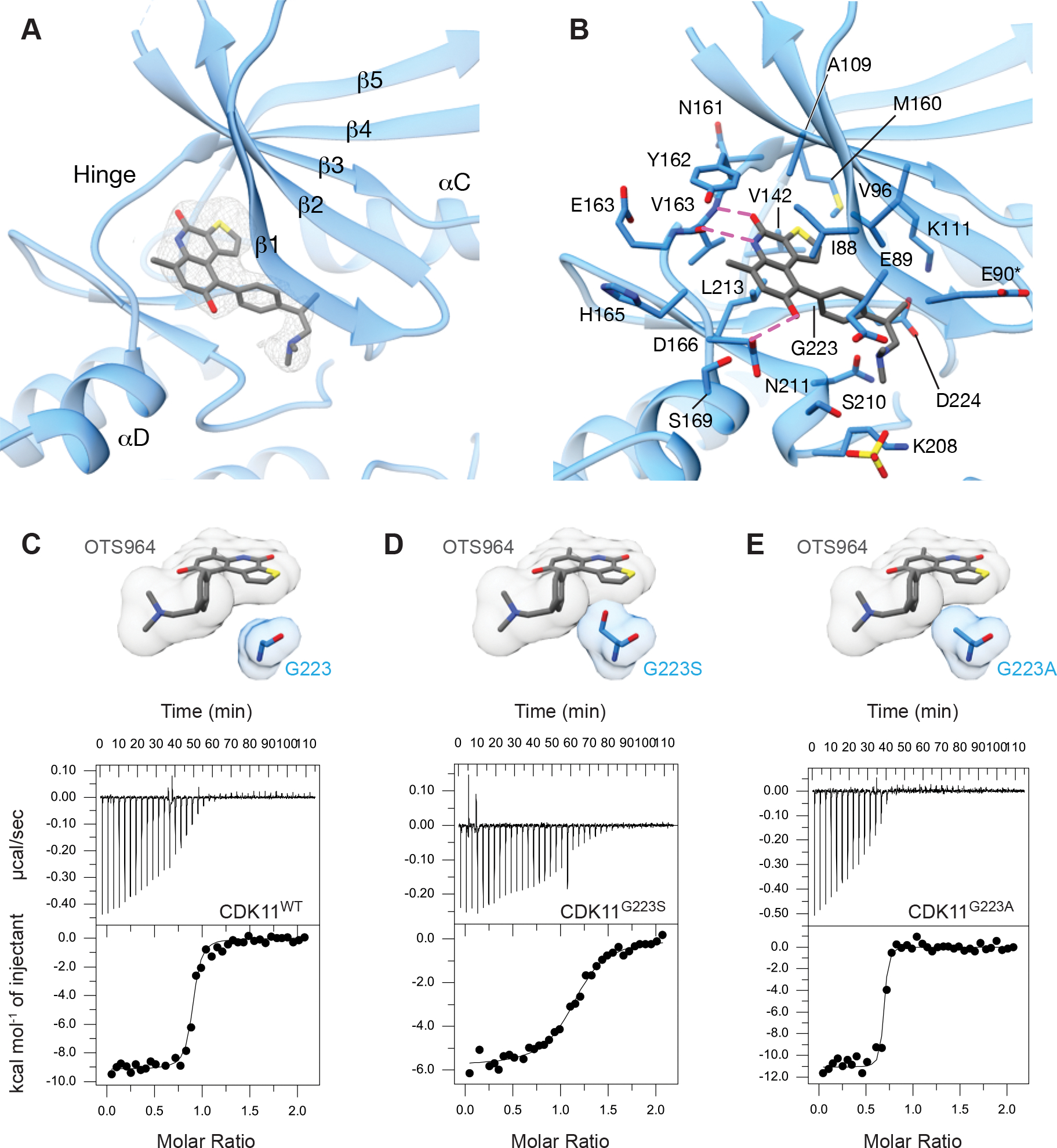
(A) View of OTS964 bound to the ATP binding pocket of CDK11 showing |Fo-Fc| electron density at 2.5 σ from prior to docking OTS964.
(B) View of OTS964 bound to the ATP binding pocket of CDK11 highlighting the position of nearby amino acids and possible hydrogen bonds in magenta. Side chains of residues marked with an asterisk were modeled for clarity.
(C) View of the position of the G223 side chain relative to OTS964, accompanied by a representative ITC (isothermal calorimetry) trace of binding between CDK11 wild-type and OTS964.
(D) View of the G223 side chain modeled as a serine (G223S), highlighting the position of the side chain relative to OTS964. Representative ITC (isothermal calorimetry) trace of binding between CDK11 G223S and OTS964.
(E) View of the G223 side chain modelled as an alanine (G223A), highlighting the position of the side chain relative to OTS964. Representative ITC (isothermal calorimetry) trace of binding between CDK11 G223A and OTS964.
