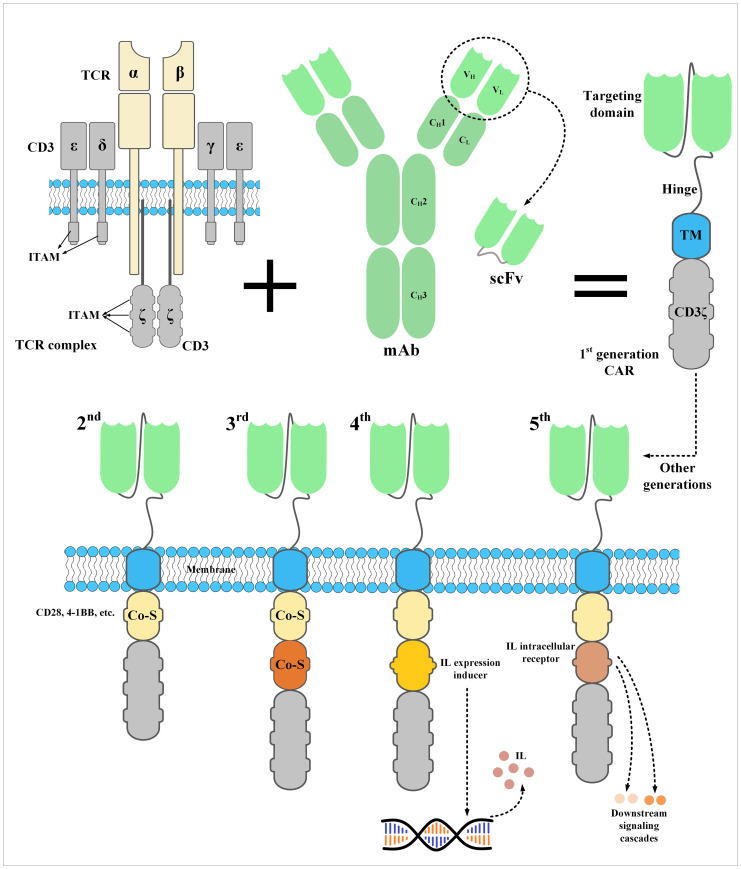
Figure 2.
The structure of a CAR and its five generations. CARs are the result of meticulous protein engineering. The targeting domain of CARs is usually derived from the single-chain fragment variable (scFv) of a monoclonal antibody. scFvs are made from the variable light chain (VL) and variable heavy chain (VH) of a monoclonal antibody fused together through a synthetic linker peptide. A spacer called hinge connects the targeting domain of CARs to their transmembrane domain, which connects the ectodomain to the endodomain. Currently, the endodomain of CARs consists of one or two costimulatory domains and an activation domain. An interleukin expression inducer domain and an interleukin intracellular receptor are also located on the endodomain of the fourth- and fifth-generation CARs, respectively. Of note, the first-generation CARs lacked a costimulatory domain which led to their inadequate in vivo persistence and weak antitumor responses. CAR, chimeric antigen receptor; Co-S, costimulatory domain; IL, interleukin; ITAM, immunoreceptor tyrosine-based activation motif; mAb, monoclonal antibody; scFv, single-chain fragment variable; TCR, T-cell receptor; TM, transmembrane domain.