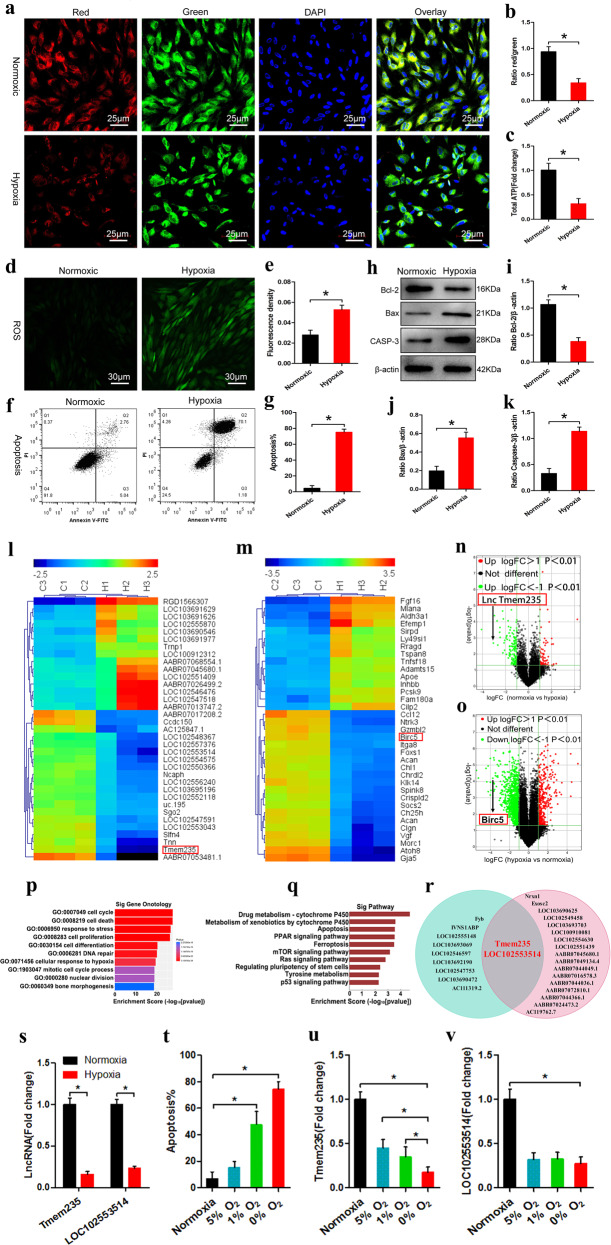
Fig. 1. The expression of Lnc Tmem235 is downregulated during hypoxia-induced apoptosis in BMSCs.
a, b JC-1 was used to detect the mitochondrial membrane potential (n = 5); bone-marrow mesenchymal stem cells (BMSCs), 5,5′,6,6′ - tetrachloro − 1,1′,3,3′ - tetraethyl - imidacarbocyanine iodide (JC-1), and 4′,6 - diamidino - 2 - phenylindole (DAPI); c ATP content (n = 6); adenosine triphosphate (ATP); d, e The content of ROS was detected by DCFH-DA (n = 5); reactive oxygen species (ROS) and 2′,7′ - dichlorofluorescin diacetate (DCFH-DA); f, g Apoptosis was detected by Annexin V/PI (n = 5); fluorescein isothiocyanate (FITC) and propidium iodide (PI); h–k The expression levels of Bcl-2, Bax, and CASP3 were analyzed by western blotting (n = 3); B-cell lymphoma 2 (Bcl-2), Bcl-2-associated X protein (Bax), and Caspase-3 (CASP-3); l Cluster analysis of lncRNAs (n = 3); m Cluster analysis of mRNAs (n = 3); n Volcano map of lncRNA expression profile (n = 3); fold change (FC), the base of logFC is 2; o. Volcano map of mRNA expression profile (n = 3); the base of logFC is 2; p GO analysis (n = 3); Gene Ontology (GO); q KEGG analysis (n = 3); Kyoto Encyclopedia of Genes and Genomes (KEGG); r Coexpression analysis and gene-position relationships were used to screen candidate lncRNAs; s The expression levels of Lnc Tmem235 and Lnc LOC102553514 were verified by qPCR (n = 6); real-time quantitative polymerase chain reaction (qPCR); t Apoptotic changes occurred as a function of hypoxia (n = 5); u Lnc Tmem235 changed with the degree of hypoxia (n = 6); v Lnc LOC102553514 changed with the degree of hypoxia. In (b, c, e, g, i–k, s–v), the data are normally distributed, and the variance is homogeneous. Data are presented as the means ± standard deviations (SDs). In (b, c, e, g, i–k, s), statistical significance was calculated by Student’s t tests; in (t–v), statistical significance was calculated by one-way ANOVA with Tukey’s post-hoc tests; *P < 0.05.