Fig. 1 |. Loss of p53 alters the neural microenvironment throughout tumour evolution.
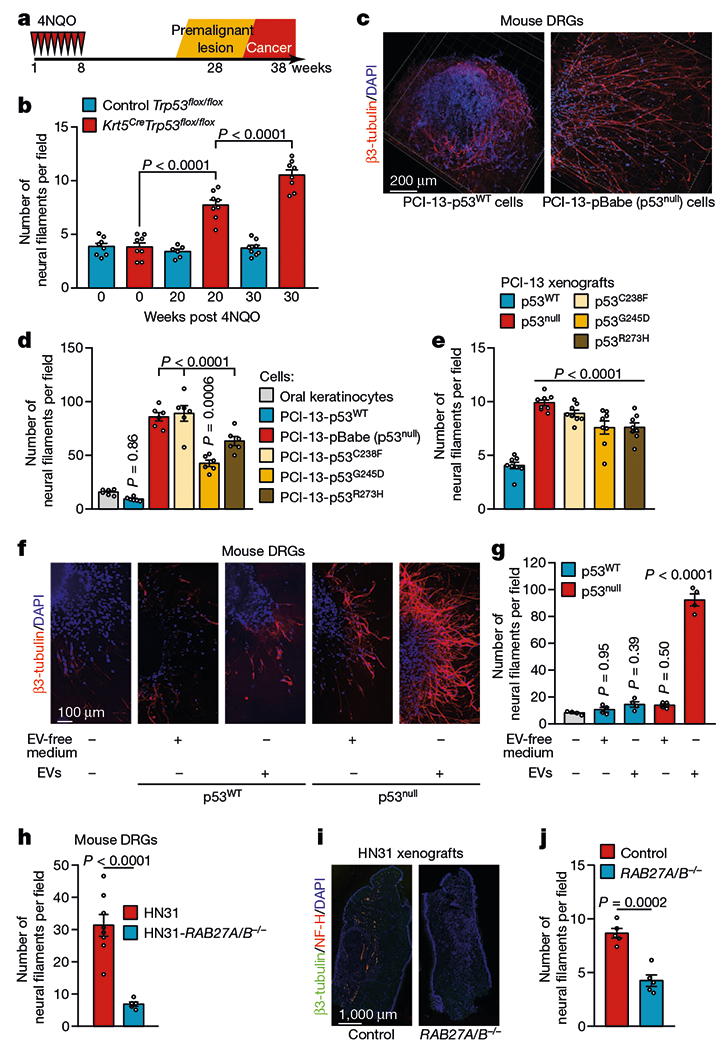
a, Tumour progression in Krt5.Cre;B6.129P2-Trp53tm1Brn/J (Krt5CreTrp53flox/flox) and B6.129P2-Trp53tm1Brn/J (Trp53flox/flox) control mice. b, Quantification of neural density in tongues from Trp53flox/flox and Krt5CreTrp53flox/flox mice immediately after the end of treatment with the carcinogen 4-nitroquinoline 1-oxide (4NQO) (normal mucosa), 20 weeks after treatment completion (low-grade lesions), and 30 weeks after treatment completion (high-grade lesions) (n = 8 except for control group at 20 weeks (n = 6)). c, Representative immunofluorescence staining of DRGs co-cultured with p53WT or p53null PCI-13 cells; data independently replicated in 12 ganglia. d, Quantification of neuritogenesis in DRGs co-cultured with p53-isogenic PCI-13 cells or normal oral keratinocytes (n = 6 biologically independent ganglia per cell line). e, Analysis of neural density in orthotopic p53-isogenic PCI-13 xenograft cells (n = 8 mice per group). f, Representative immunofluorescence of in vitro neuritogenesis in DRGs treated with soluble factors (EV-depleted conditioned medium) or EVs from p53-isogenic PCI-13 cells; data independently replicated in 20 ganglia. g, In vitro quantification of neuritogenesis (n = 4 biologically independent ganglia per condition). h, Quantification of neuritogenesis in freshly collected DRG cultured with conditioned medium from HN31 RAB27A/RAB27B-isogenic human OCSCC cells (n = 8 and n = 5 biologically independent ganglia for HN31 RAB27A−/−RAB27B−/− and HN31 RAB27A+/+RAB27B+/+ cells respectively). i, Representative immunofluorescence montage of glossectomy specimens derived from mice 3 weeks after injection of HN31 RAB27A/RAB27B-isogenic OCSCC cells; data independently replicated in 10 mice. j, In vivo analyses of neural density (n = 5 mice per group). Unpaired two-tailed t-test (h, j) and one-way ANOVA with Tukey multiple comparisons (b, d, e, g). Bar graphs represent mean ± s.e.m.
