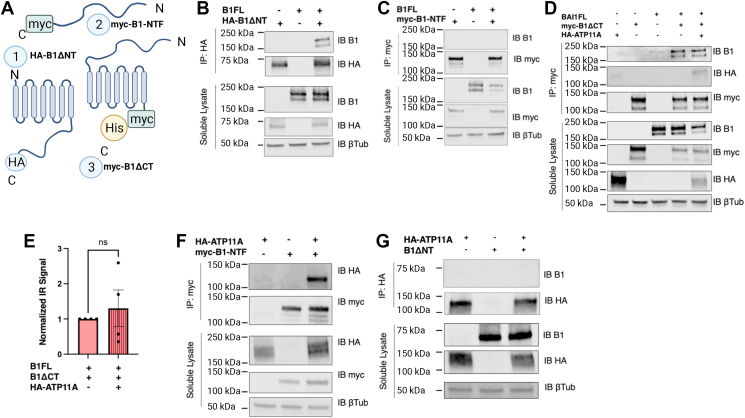
Figure 4.
B1 forms PS-independent multimers and also interacts with ATP11A.A, B1 schematics: B1 constructs used to evaluate multimer formation included (1) HA-tagged B1ΔNT, (2) myc-tagged B1-NTF, (3) His/myc-tagged B1ΔCT. B, Immunoprecipitation (IP) of HA-B1ΔNT resulted in co-IP of B1FL (n = 3). C, IP of myc-tagged B1-NTF did not result in any co-IP of B1FL (n = 3). “Soluble lysate” refers to the detergent-solubilized cell samples, which serve as transfection controls. D, IP of His/myc-B1ΔCT resulted in co-IP of B1FL in a manner that was not affected by coexpression with ATP11A (n = 4). Intriguingly, ATP11A itself also coimmunoprecipitated with His/myc-B1ΔCT. E, quantification of effect of ATP11A on B1FL-His/myc-B1ΔCT dimer formation: HA-ATP11A coexpression with B1FL and His/myc-B1ΔCT did not disrupt the ability of B1 to form multimers (normalized mean ± SEM is shown, unpaired t test, n = 4). F, IP of myc-tagged B1-NTF resulted in co-IP of HA-ATP11A (n = 3). G, IP of HA-ATP11A did not result in any detectable co-IP with B1ΔNT (n = 4). B1FL, full-length B1; HA, hemagglutinin; PS, phosphatidylserine.