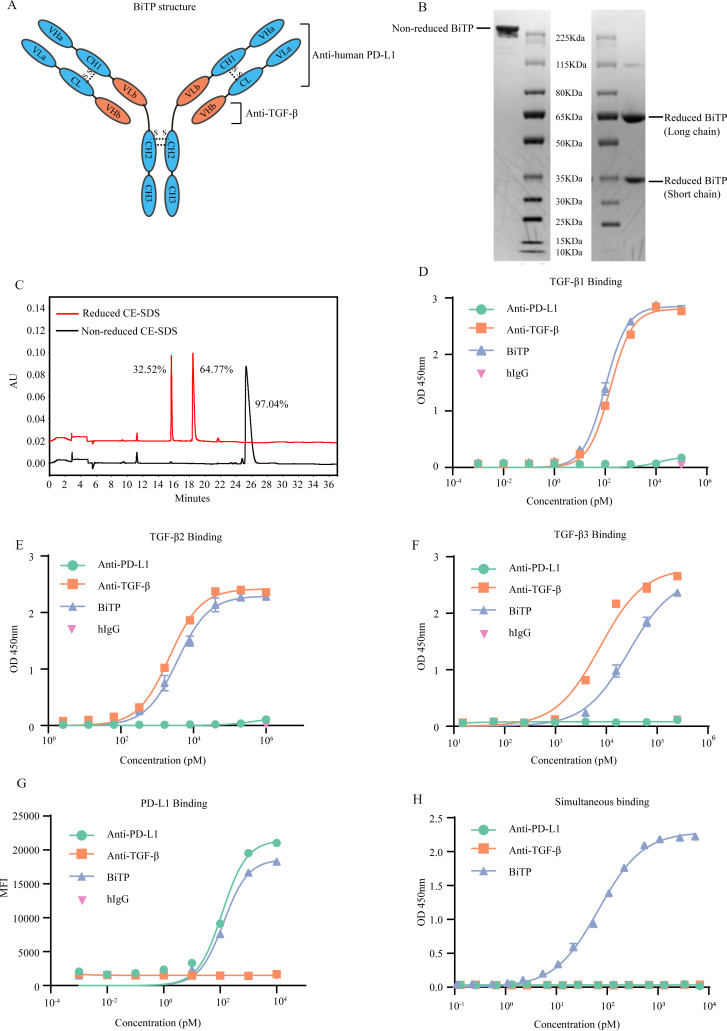
Figure 1.
The structure and binding affinity of BiTP. (A) The structure of BiTP. BiTP is an IgG1/IgG2 hybrid antibody, containing IgG2-derived CH2 domain and IgG1-derived CH3 domain. The VLa, CL, VHa, and CH1 are derived from the corresponding domains of anti-PD-L1. The VHb and VLb domains are derived from the corresponding domains of anti-TGF-β. (B) Non-reduced and reduced SDS-PAGE. (C) Non-reduced and reduced CE-SDS. (D–F) The binding affinity to TGF-β1. BiTP was captured by plate-coated TGF-β. The affinity was determined by ELISA. (G) The binding affinity to PD-L1. Antibodies were incubated with H358 cells. The binding affinity was measured by mean fluorescence intensity in flow cytometry assay. (H) The simultaneous binding to PD-L1 and TGF-β1. BiTP was captured by precoated TGF-β1. Then, PD-L1-HRP was added, and the simultaneous binding was detected by ELISA. CE-SDS, capillary electrophoresis-sodium dodecyl sulfate; MFI, mean fluorescence intensity; TGF-β, transforming growth factor-beta.