Figure 6. UNC‐51 phosphorylates PHIP‐1 at Ser‐112.
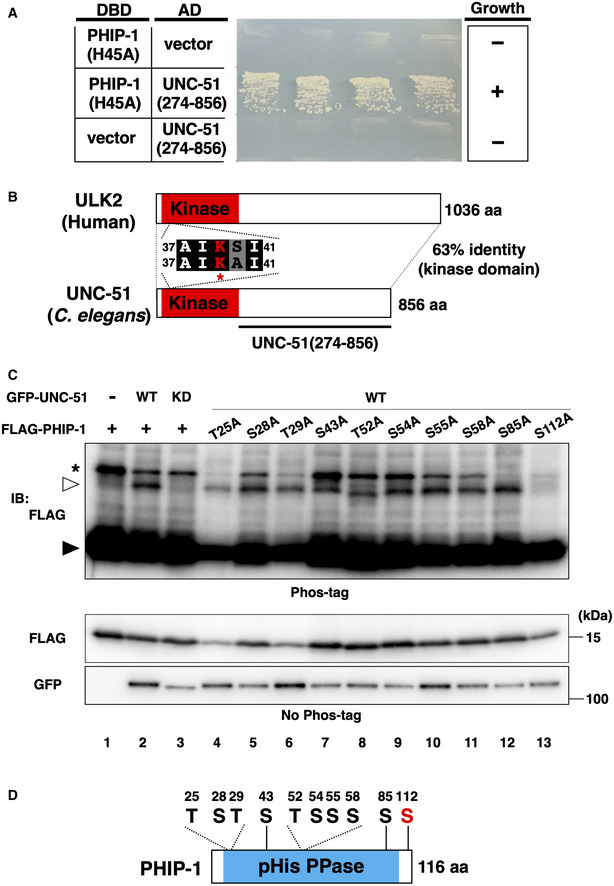
- PHIP‐1 interaction with UNC‐51 by yeast two‐hybrid assay. The reporter strain PJ69‐4A was co‐transformed with expression vectors encoding GAL4 DBD‐PHIP‐1(H45A) and GAL4 AD‐UNC‐51(274–856), as indicated. Yeast strains carrying the indicated plasmids were cultured on a selective plate lacking histidine and containing 5 mM 5‐aminotriazole for 4 days.
- UNC‐51 structure. Schematic diagrams of UNC‐51 and human ULK2 are shown. The kinase domain is shown in red. The catalytic lysine and four flanking amino acids are shown. Identical and similar residues are highlighted with black and gray shading, respectively. The unc‐51(ks49) mutation is a splice site mutation, which significantly reduces the unc‐51 mRNA level.
- UNC‐51 phosphorylates PHIP‐1 at Ser‐112. COS‐7 cells were co‐transfected with Flag‐PHIP‐1 (WT or mutants) and GFP‐UNC‐51 [WT or ∆AIKAI (KD)], and cell lysates were analyzed using Phos‐tag SDS–PAGE. Total lysates were immunoblotted (IB) with antibodies, as indicated. Filled and open arrowheads indicate unmodified and phosphorylated PHIP‐1, respectively. Asterisk indicates nonspecific band.
- Schematic representation of the 10 Ser/Thr residues and domain structure in PHIP‐1.
