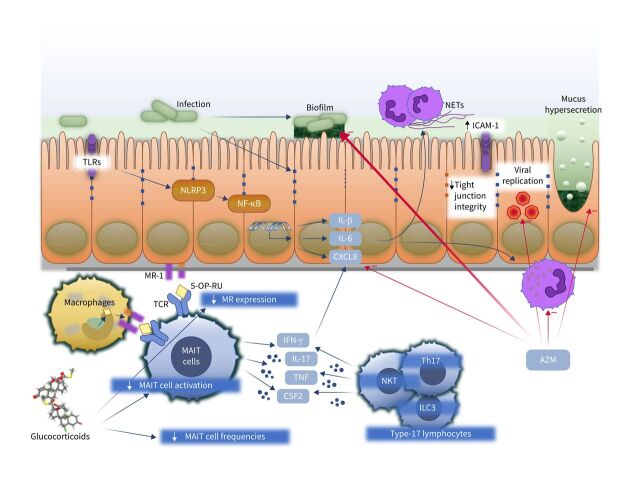
FIGURE 1.
Immunological consequences of non-typeable Haemophilus influenzae (NTHi) infection. Initial acquisition of NTHi may be enhanced by glucocorticoid therapy, which, for example, suppresses the protective mucosal-associated invariant T-cell (MAIT)–major histocompatibility complex class I-related (MR1) axis by reducing MAIT cell frequencies, suppressing MAIT cell activation and reducing expression of MR1. NTHi infection leads to stimulation of pathogen receptors including toll-like receptors (TLRs) and the NLR family pyrin domain containing 3 (NLRP3) inflammasome, generating release of proinflammatory cytokines including interleukin (IL)-1β, IL-6 and C-X-C motif chemokine ligand 8 (CXCL8). These recruit and activate neutrophils. Neutrophil activation causes release of neutrophil extracellular traps (NETs), which contribute to mucus hyperviscosity and the development of NTHi biofilms. NTHi also reduces expression of tight-junction proteins and induces upregulation of intracellular cell adhesion molecule 1 (ICAM-1), the receptor for many respiratory viruses, including most rhinoviruses, favouring viral replication. Activation of innate and adaptive type-17 lymphocytes including MAIT, type 17 T-helper cells (Th17), natural killer T-cells (NKT) and type 3 innate lymphoid cells (ILC3) leads to production of interferon γ (IFN-γ), tumour necrosis factor (TNF), colony-stimulating factor 2 (CSF2) (also known as granulocyte–macrophage CSF) and IL-17, favouring neutrophil recruitment. The macrolide azithromycin (AZM) suppresses several features of neutrophilic asthma, including reducing CXCL8 production, inhibiting biofilms, direct antiviral activities, reducing mucus hypersecretion and suppressing neutrophil oxidative burst and chemokine production and survival. TCR: T-cell receptor; 5-OP-RU: 5-(2-oxopropylidenamino)-6-D-ribitylaminouracil; NF-kB: nuclear factor kB.